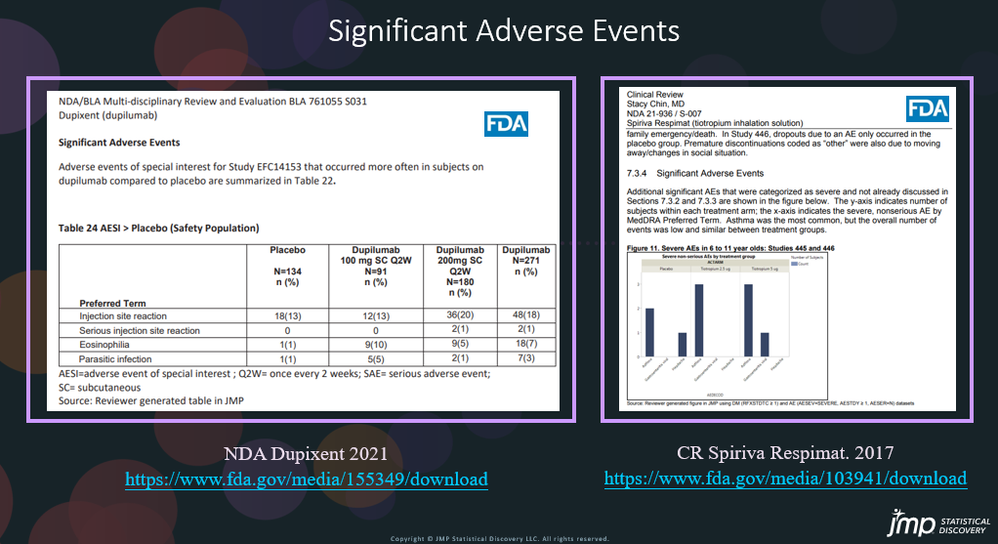
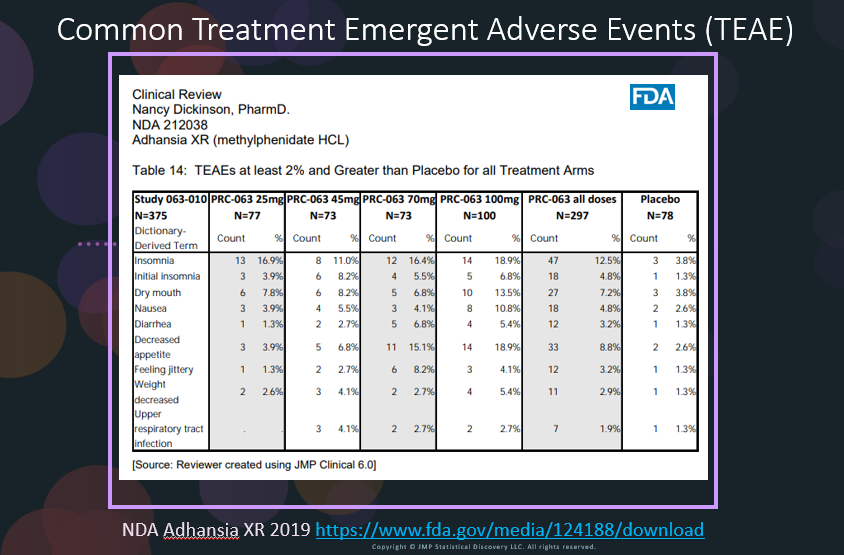
Safety Results IIIb: Significant Adverse Events by AE Risk Report in JMP® Clinical
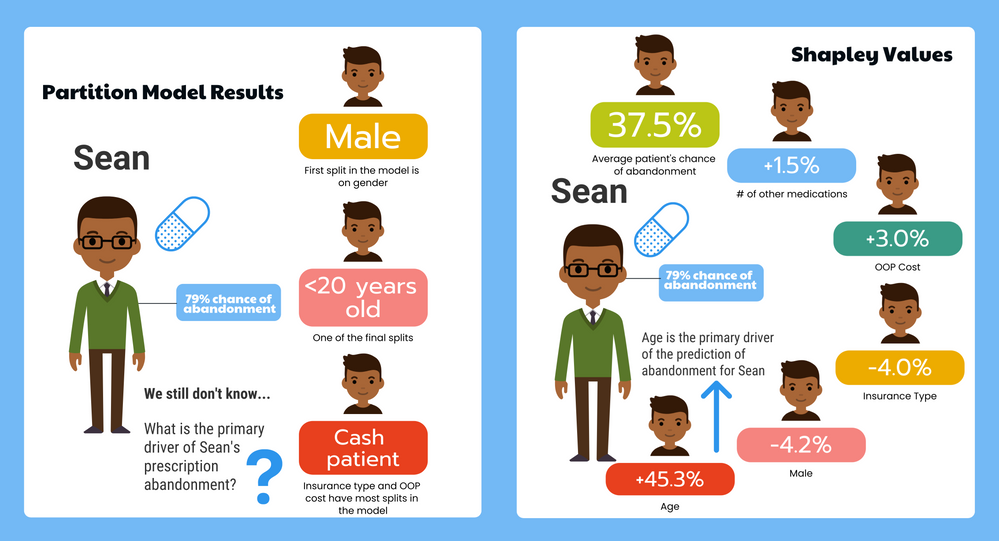
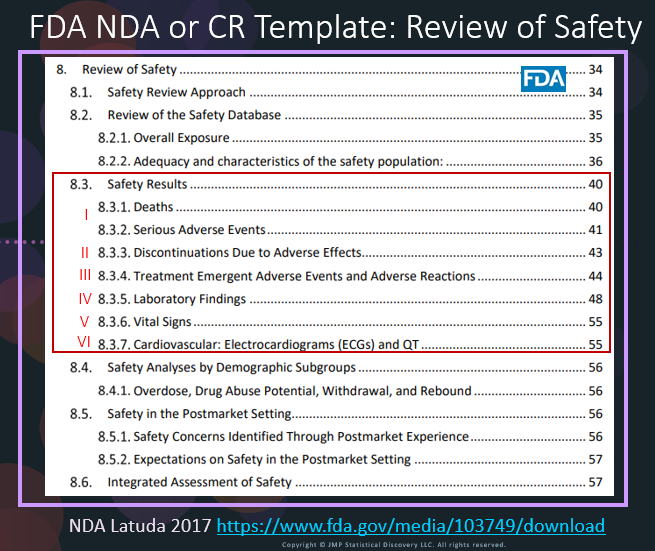
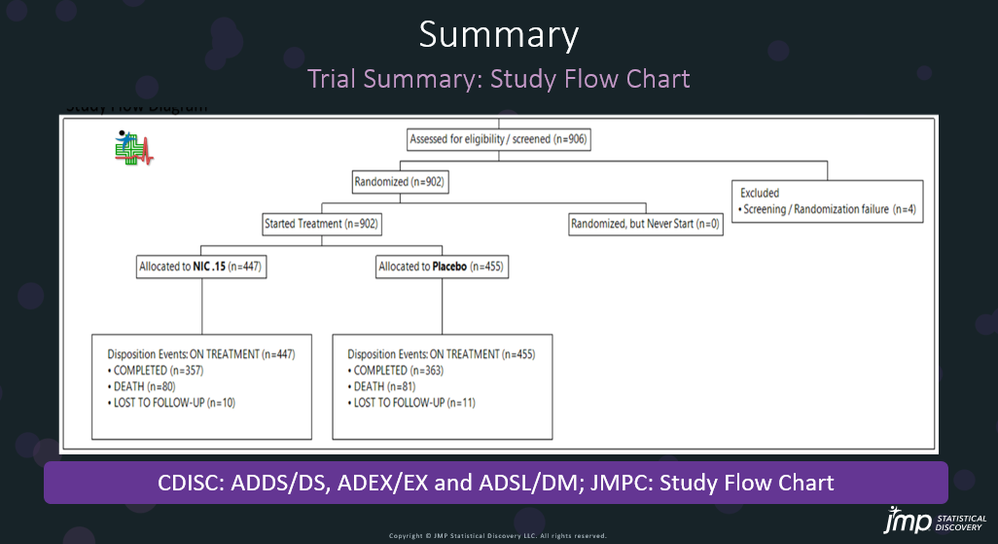
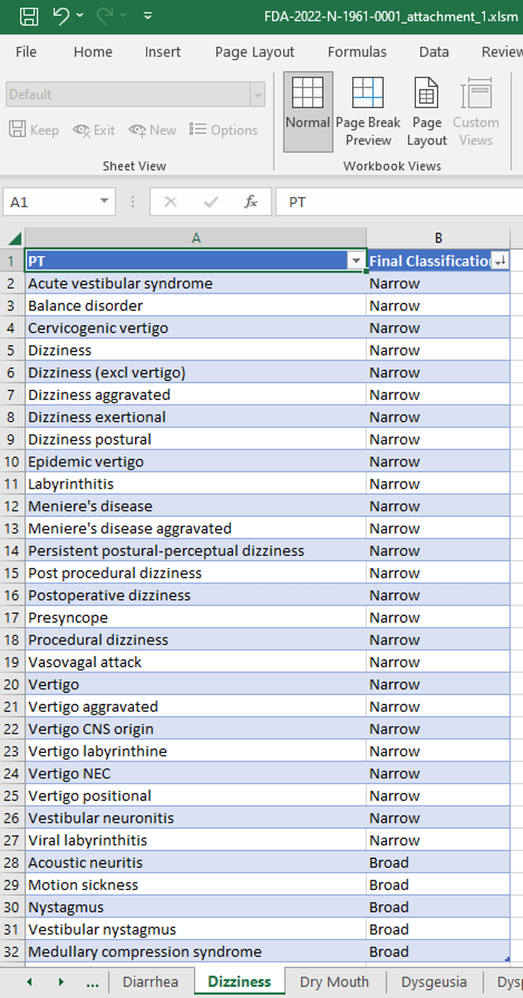
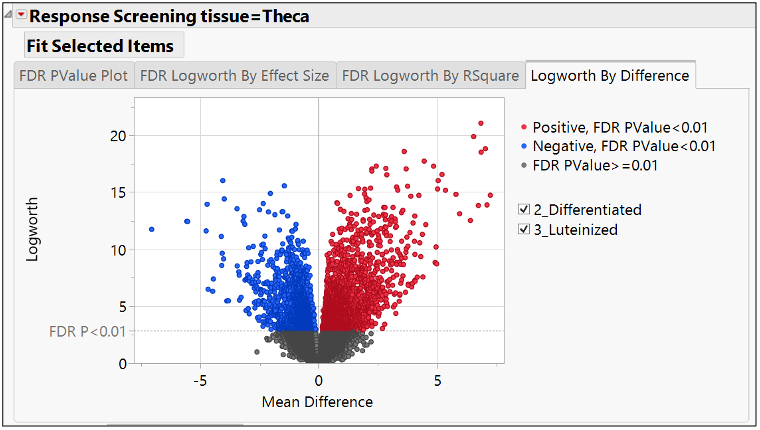
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Clinical Reviews) templates to show how drug safety can be evaluated. My previous blogs demonstrated how to capture SAEs using Adverse Events Narrative3, and Discontinuations Due4 to AEs and TEAE5 with Adverse Events Distribution. There is a final step...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP


 gail_massari
gail_massari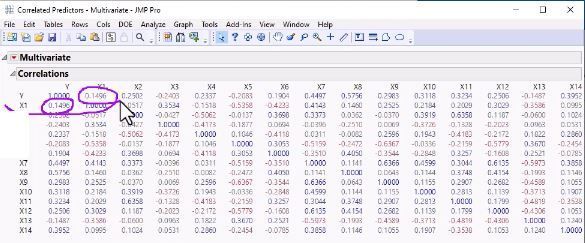
 Valerie_Nedbal
Valerie_Nedbal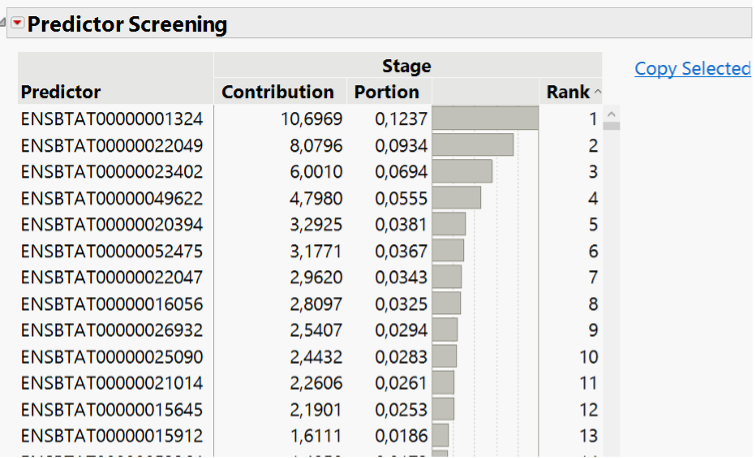
 Dahlia_Watkins
Dahlia_Watkins

 Duane_Hayes
Duane_Hayes
 tonya_mauldin
tonya_mauldin



 KristenBradford
KristenBradford