An interview with political science major Megan Tengan on analyzing text data from Twitter to gauge public sentiment about critical race theory and Covid policy in Hawai’i
Featured Research
 Meg_Hermes
Meg_Hermes
Featured Research
 Meg_Hermes
Meg_Hermes

Featured Research
 Meg_Hermes
Meg_Hermes

Advocating for Analytics at Syngenta
 Meg_Hermes
Meg_Hermes

Advocating for Analytics
 Meg_Hermes
Meg_Hermes

An analytics advocate interview
 Meg_Hermes
Meg_Hermes

An analytics advocate interview
 Meg_Hermes
Meg_Hermes

An analytics advocate interview
 Meg_Hermes
Meg_Hermes

An analytics advocate interview
 Meg_Hermes
Meg_Hermes

The new version of JMP Pro encompasses nearly all functionality formerly available in JMP Genomics.
 Valerie_Nedbal
Valerie_Nedbal
Get a better look at the underlying "plumbing" layer that powers the porcelain layer.
 LisaG
LisaG

The Distribution platform can be used to explore the distribution of a single variable. This is a rich platform that allows many different visuals, statistics, and tests on variables. This blog post details the new features to the Distribution platform in version 16 of JMP.
 tonya_mauldin
tonya_mauldin

Is there anything more magical than a beautifully decorated Christmas tree?
 sabrinafong8
sabrinafong8
Learn what the ! (NOT) operator is and see examples of how you can make use of it in your JSL code.
 nickholmes13
nickholmes13

Do you do any of the following Script JSL as well as other languagesUse git or other version control software for you JSL scriptsAlready use VSCode for other thingsJust want to code JSL in something that feels more like an IDE Then I've got some good news! YAY!
 vince_faller
vince_faller

Ever been confused when trying to add numeric values and ended up with a missing value as the result? Maybe you discovered the Sum() function to get around the problem but never really understood why it worked. In this blog post, I explain the Add() function, plus (+) operator, and the Sum() function to help you get the results you need from your script or column formula.
 Wendy_Murphrey
Wendy_Murphrey

The first Mastering JMP Developer Tutorial is Oct. 21. Ryan Lekivetz talks about using covariates when designing experiments. Ryan makes learning about designing experiments fun, and recently did a small experiment with his masked but recognizable co-workers in North Carolina.
 gail_massari
gail_massari

Starting Nov. 4, Developer Clay Barker will present three Developer Tutorials.
 gail_massari
gail_massari

Advocating for analytics at Omya Craig DePorter of Omya
 Meg_Hermes
Meg_Hermes

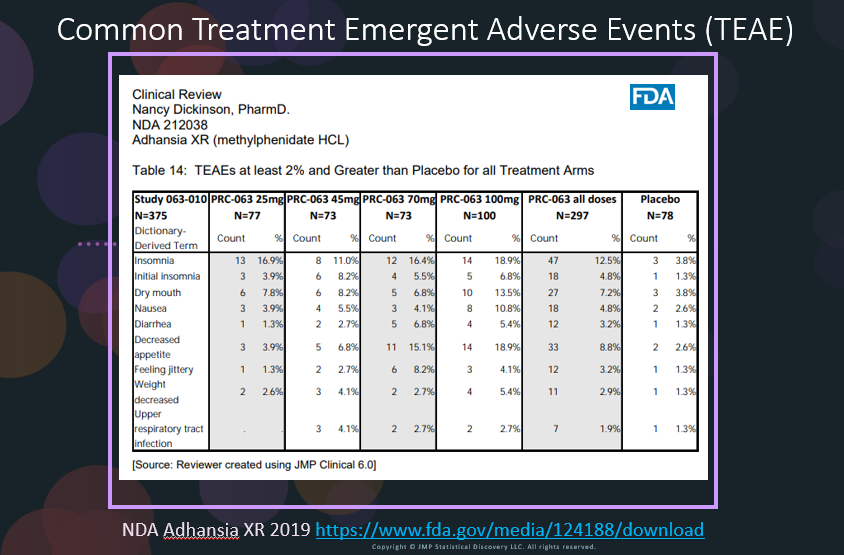
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Clinical Reviews) templates to show how drug safety can be evaluated. My previous blogs demonstrated how to capture SAEs using Adverse Events Narrative3, and Discontinuations Due4 to AEs and TEAE5 with Adverse Events Distribution. There is a final step...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
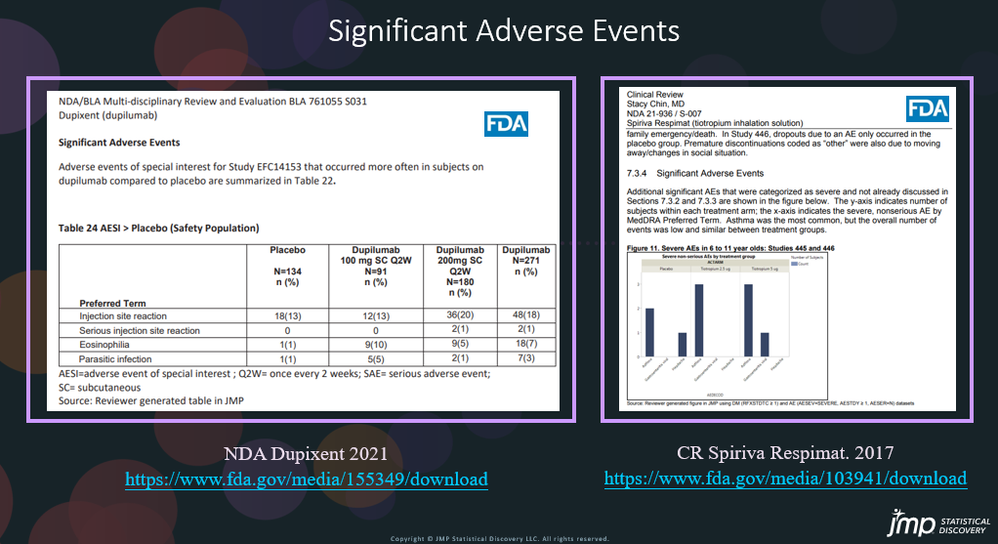
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Clinical Reviews) templates to show how drug safety can be evaluated. My previous blogs demonstrated how to evaluate SAEs by Adverse Events Narrative3 and Discontinuations Due to AEs4 by Adverse Events Distribution. We'll now look at the third step for...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP