What's new in JMP® Live 18?
Organizations rely on JMP Live because it enriches how discoveries are shared, how collaboration happens, and how data-driven decisions are made. JMP ...
 dieter_pisot
dieter_pisot
Organizations rely on JMP Live because it enriches how discoveries are shared, how collaboration happens, and how data-driven decisions are made. JMP ...
 dieter_pisot
dieter_pisot
JMP Clinical 18 (planned for release March 2024) has several new and important features, including new reports, a new method for querying the study da...
 SamGardner
SamGardner
Another way to navigate JMP reports? Yes, please!
 Wendy_Murphrey
Wendy_Murphrey
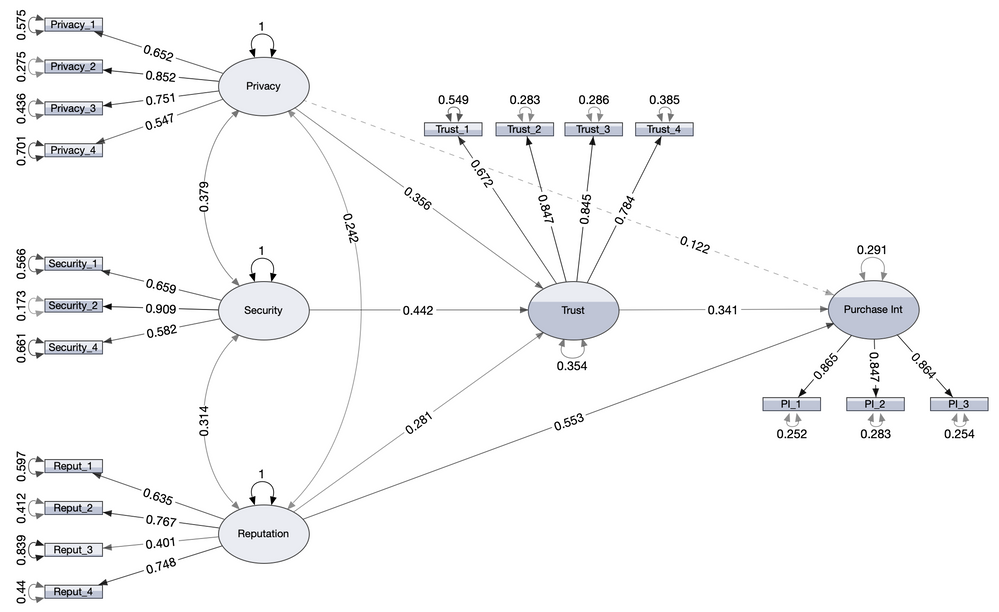
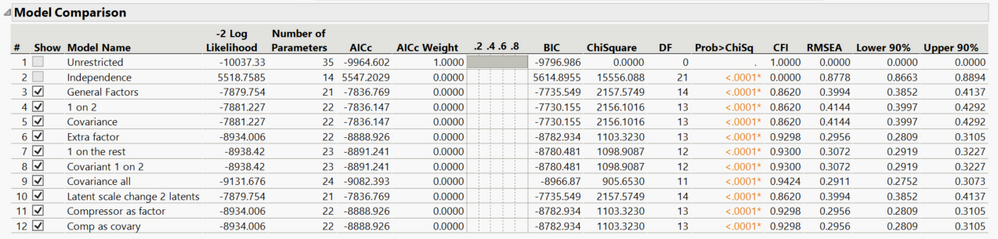
My really fun project and collection of exciting tricks are embodied in the structural equation modeling platform in JMP Pro. I’ve written before abou...
 LauraCS
LauraCS
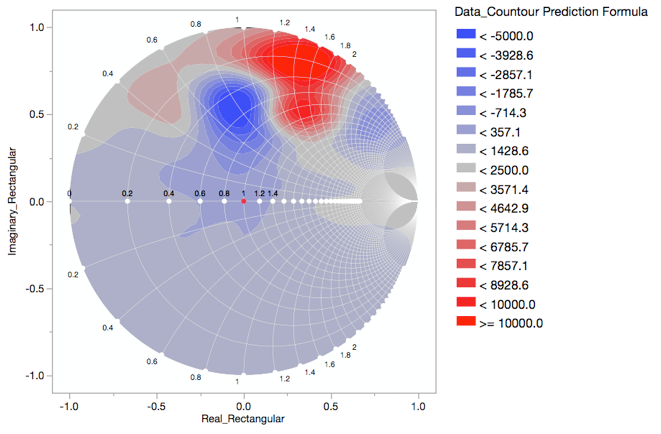
It started with a "quick" question about how to make a Smith Chart.
 MikeD_Anderson
MikeD_Anderson
In this month's post, I carry on the work performed in my last post by constructing structural equation models for the Tennessee Eastman simulator. Ma...
 jordanwalters
jordanwalters
This article originally appeared in JMPer Cable, Issue 26, Winter 2010by Joseph Morgan joseph.morgan, JMP Senior Software Developer, SASNote: The JSL ...
 gail_massari
gail_massari

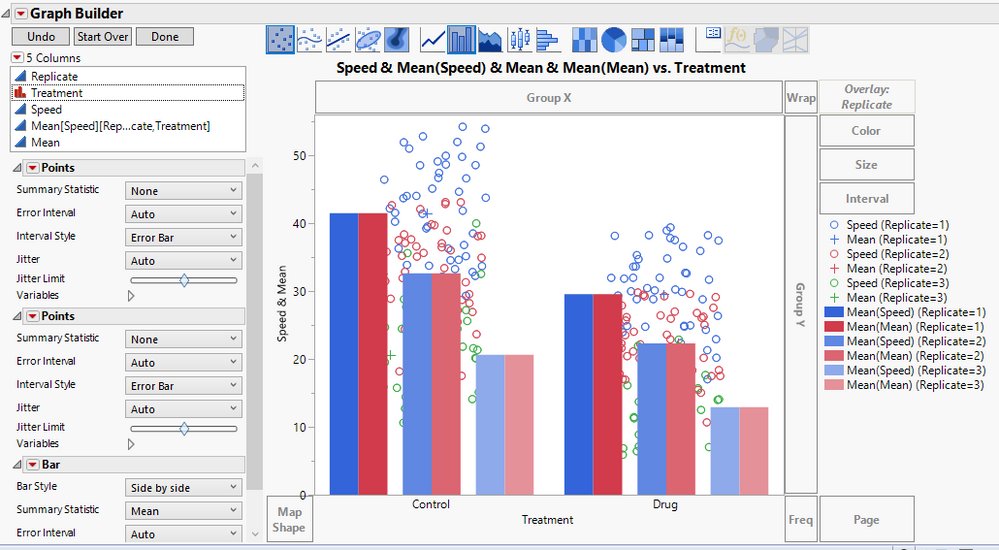
See how to reproduce a SuperPlot using JMP with an emphasis on presenting observation-level variability and experimental reproducibility simultaneousl...
 laura_archer
laura_archer
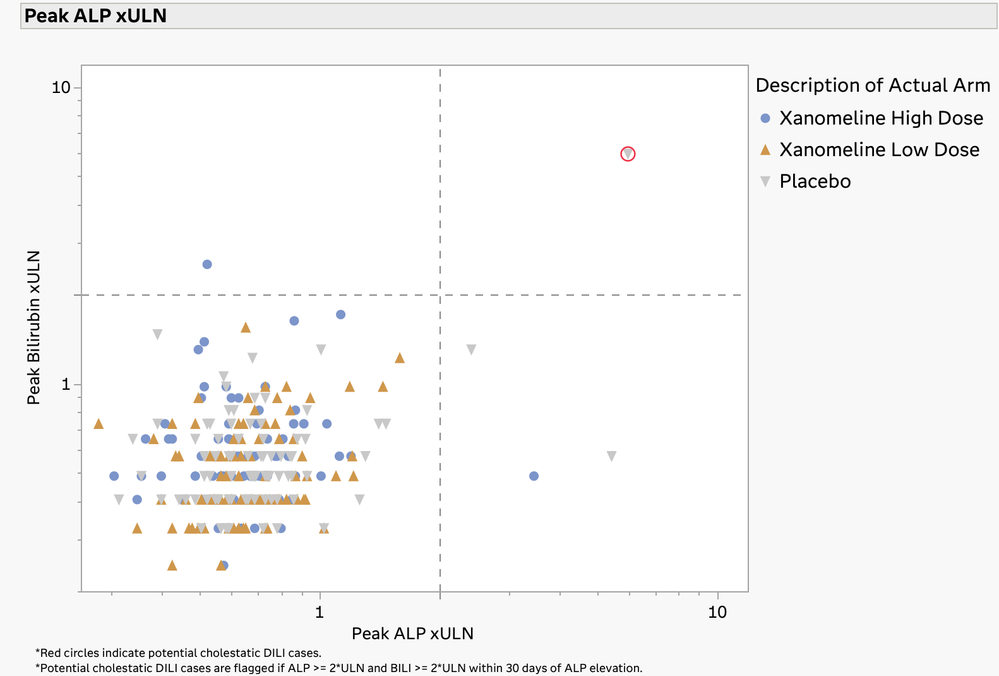
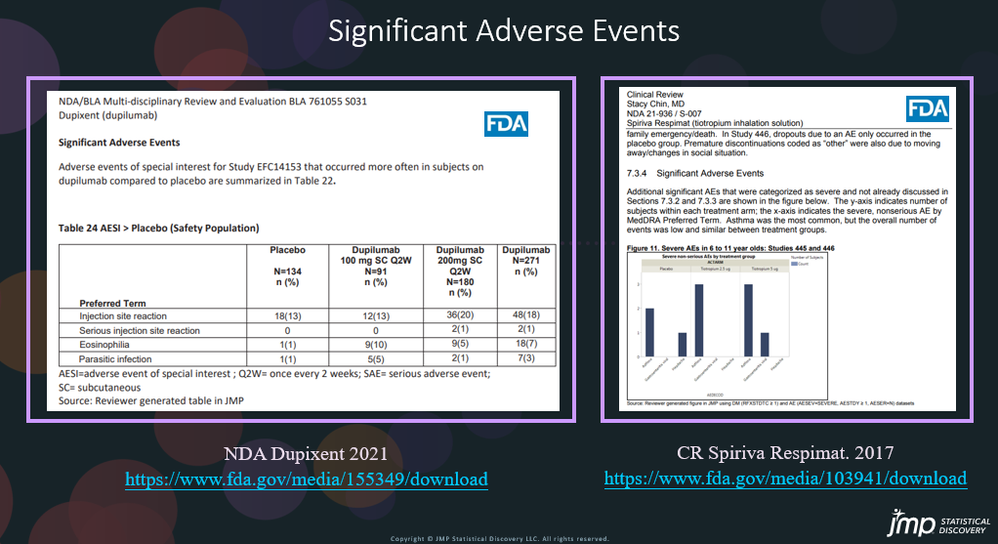
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Cl...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
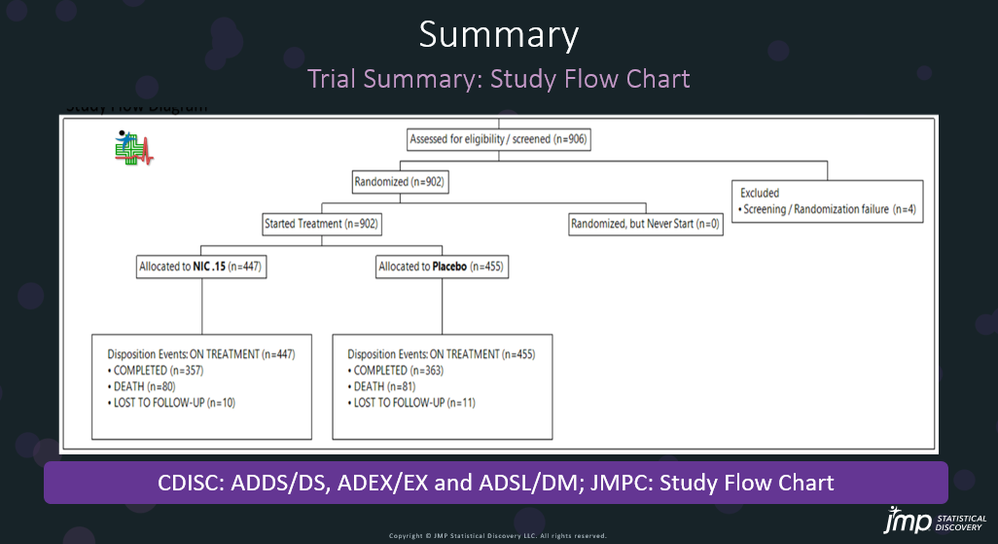
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Cl...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
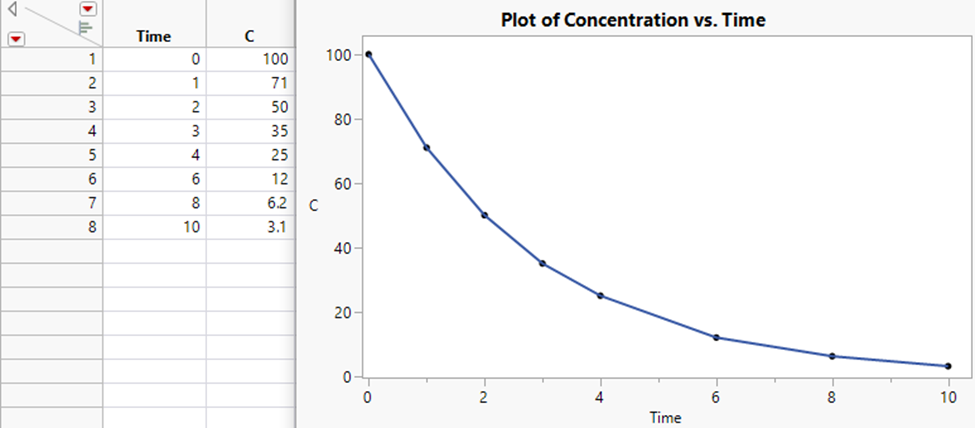
A common question users ask is how to use JMP to find the area under a curve. In this blog post, I’ll review a few possible ways to do so in JMP.
 sseligman
sseligman
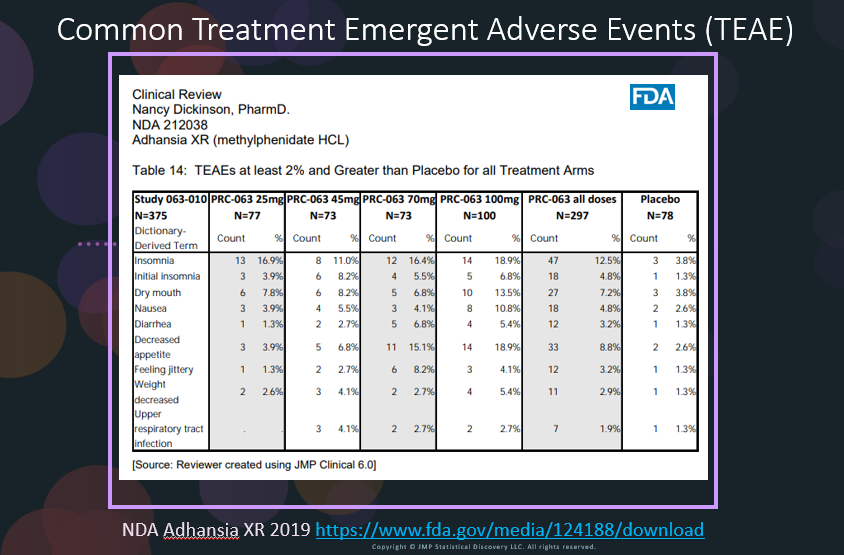
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Cl...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
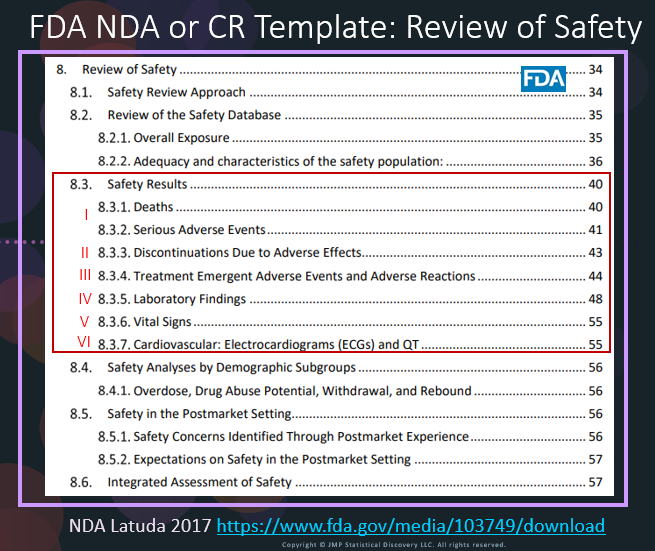
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Cl...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP

Before we can understand clinical trial data in detail, we need to understand the protocol or trial design to get a big picture of the clinical trial ...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
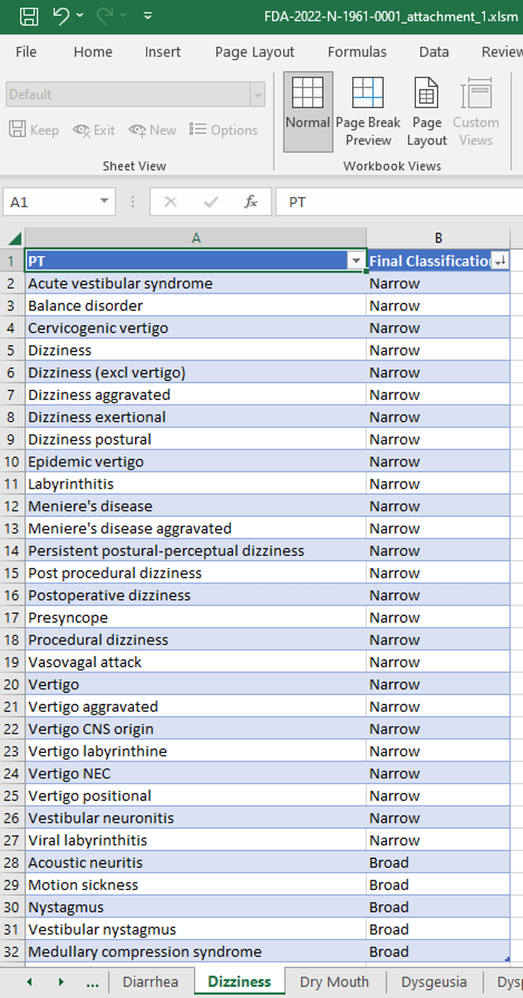
The FDA announced the release of the FDA Medical Query (FMQ) and Algorithmic FDA Medical Queries (AFMQ) at the Duke Margolis-FDA Workshop: Advancing P...
 Wenjun_Bao_JMP
Wenjun_Bao_JMP
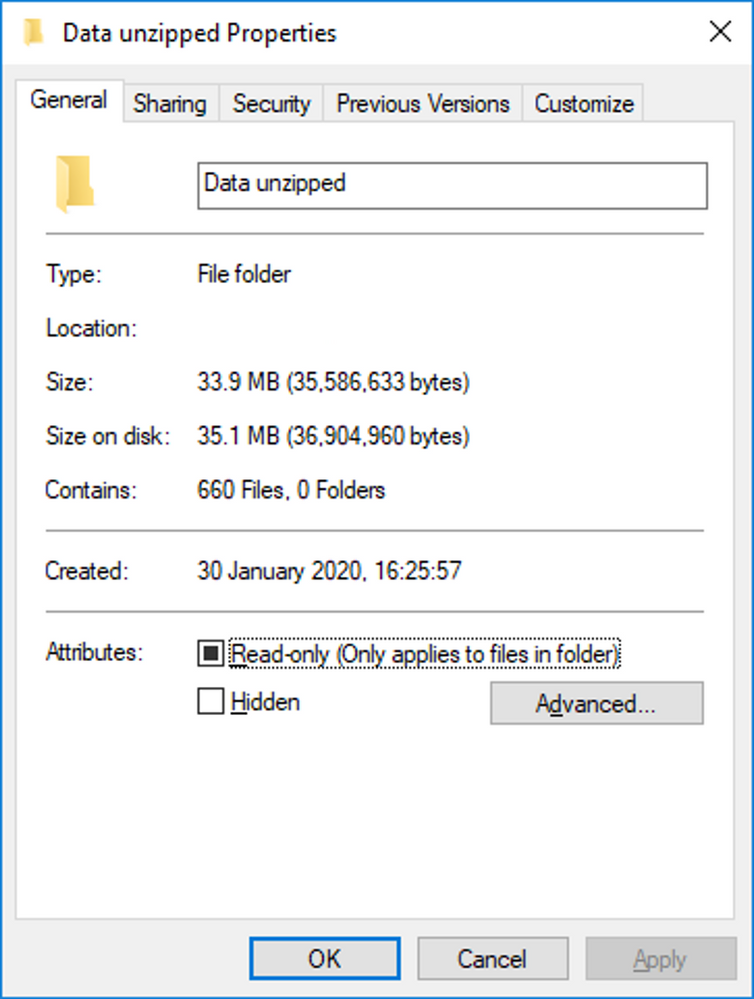
I needed to import and stack 660 XML files from my exercise activity tracking app. It's easy with the XML Import Wizard and Import Multiple Files in J...
 Phil_Kay
Phil_Kay
Learn how to convert number between different bases.
 paul_vezzetti
paul_vezzetti

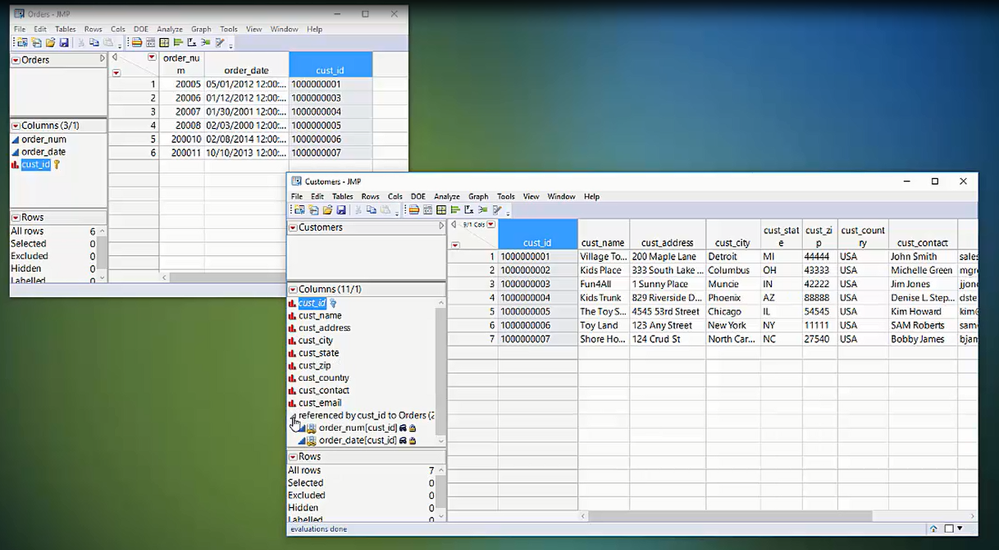
Get a video introduction to the basic concepts of Virtual Join.
 Dahlia_Watkins
Dahlia_Watkins
Creating a library of peer-reviewed articles focused on the implementation of CDISC standards.
 Wenjun_Bao_JMP
Wenjun_Bao_JMP

 MikeD_Anderson
MikeD_Anderson
