JMP Blog
A blog for anyone curious about data visualization, design of experiments, statistics, predictive modeling, and more- JMP User Community
- :
- Blogs
- :
- JMP Blog
- :
- JMP確定性篩選實驗設計(DSD)在生物製藥製程特性研究中的應用
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
生物技術是21世紀的戰略新興行業,大力發展以生物技術為核心的生物製藥行業不僅有利於促進創新型經濟轉型,更是推進健康中國建設,提高人民健康水準的必由之路。近年來,隨著藥品審評審批制度的改革和完善,新藥獲批的數量增多,速度加快,也給生物製藥企業帶來新的挑戰,即如何迅速將複雜的生物製藥生產製程從小試放大到生產,建立可靠穩定的生產控制策略,持續生產出安全有效的藥品。
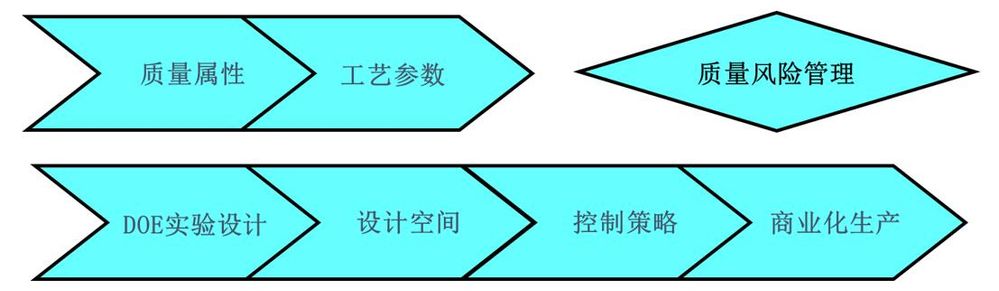
面對這一難題,國內外大型製藥企業普遍依據ICH 相關指導原則,以品質源於設計(Quality by Design, QbD)的核心理念,通過廣泛使用實驗設計(Design of Experiment, DOE),解決生物藥物生產製程中製程參數多,交互作用複雜的難題。在小規模詳盡DOE實驗的基礎上,建立生產製程參數與品質屬性的關聯關係,充分理解生產製程的特性,從而有效地保證大規模生產的順利實施詳見圖1。
圖1.依據ICH指導原則進行藥品研發和生產的流程圖
使用DOE方法可以用較少的實驗,研究多個參數的關係。傳統DOE實施方法首先對大量參數進行解析度較低的篩選實驗設計,根據實驗結果還可能需要第二輪的擴增設計或反應曲面實驗設計。生物藥生產製程參數多,常包含需要通過反應曲面設計才能分辨清楚的高階效應,此外,還會面臨實驗材料成本和時間緊張的壓力。這些實際困難決定了使用傳統的DOE實驗設計方法進行生物藥物的研發具有很大的挑戰。而JMP軟體所特有的確定性篩選實驗設計(Definitive Screening Design, DSD)具備實驗次數少、解析度高、節省時間等優點,為解決這一問題提供了極大的便利。下面通過具體實例進行說明。
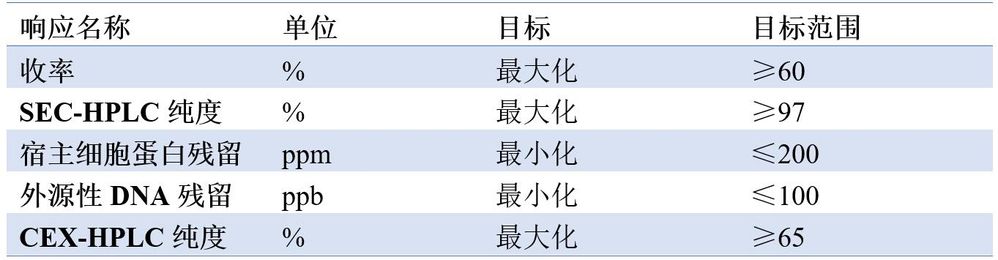
單株抗體的生產過程包括上游細胞培養、下游純化和產品灌裝3個部分。陽離子交換層析是下游純化生產中精細純化的重要步驟,通過陽離子交換層析可有效去除聚合體、電荷變異體、宿主細胞蛋白殘留和外源性DNA殘留等雜質,這些雜質均為關鍵品質屬性(Critical Quality Attribute, CQA)。通過實驗設計方法,研究陽離子交換層析製程參數對CQA的影響關係,制定合適的控制策略。陽離子交換層析考察的反應指標見表1。
表1.陽離子交換層析考察的反應指標
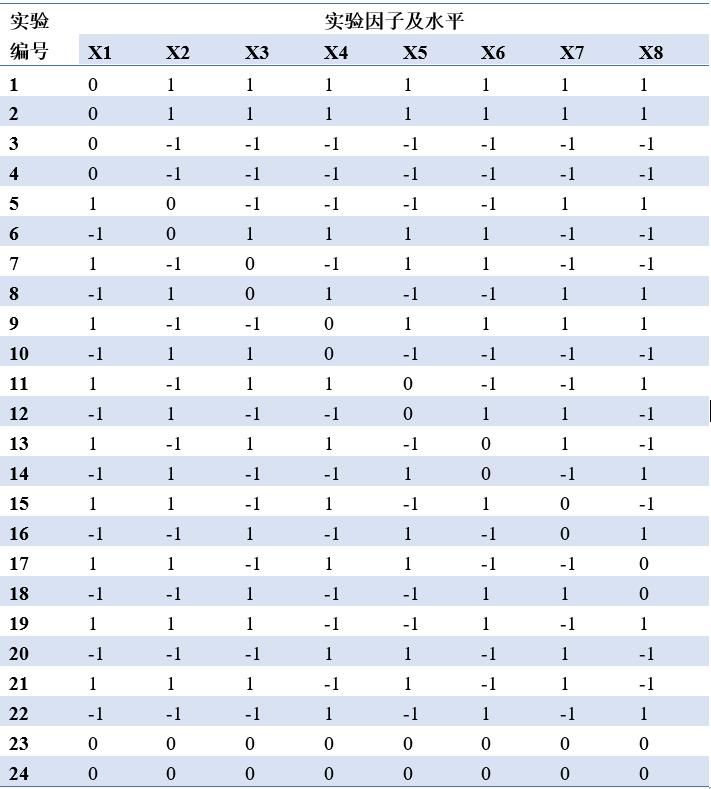
陽離子交換層析製程過程包括近20項製程參數,根據其他專案的知識和經驗,評選層析流速、載量等可能對純化結果有影響的8個製程參數進一步實驗考察。通過JMP 軟體DSD實驗設計制定研究方案。所有製程參數均為連續性變數,不需要劃分區組,JMP軟體預設生成一組包含21個實驗運行的方案。研發人員又增加了3個條件下的各1次重複實驗,以考察實驗過程的系統誤差,總共進行了24次實驗,方案詳見表2。
表2.陽離子交換層析實驗方案
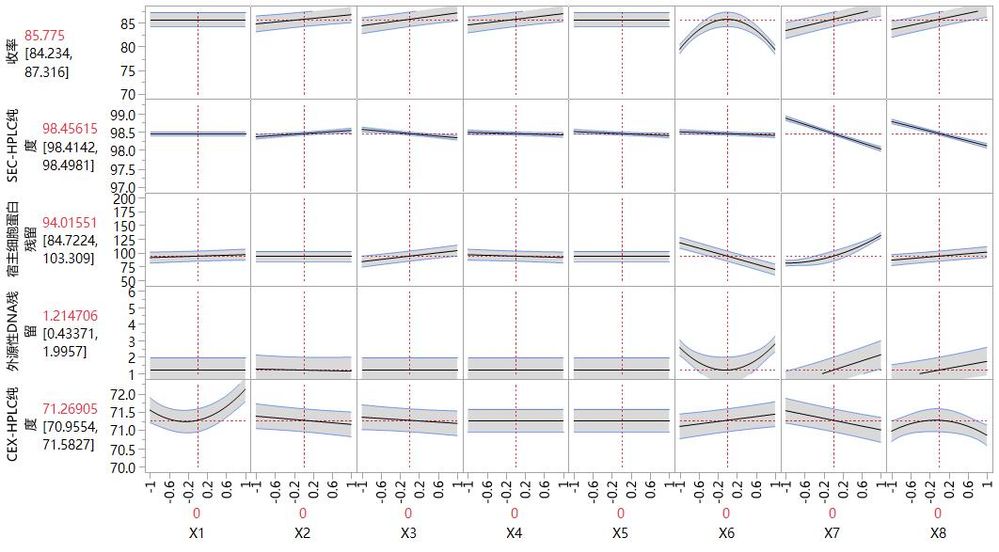
按照實驗方案隨機運行實驗,並收集檢測資料。 由於DSD利用較少的實驗次數對大量的因數和效應進行考察,無法對DSD資料直接進行標準最小平方分析,可以利用JMP軟體特有的“擬合確定性篩選”方法,或通過“擬合模型”中的“逐步迴歸”擬合方法。研發人員通過逐步擬合的方法分別對收率、SEC-HPLC純度等5個回應成功建立了回歸模型,預測刻畫器結果見圖2。
圖2. 陽離子交換層析實驗設計預測刻畫器結果
根據以上模型,陽離子製程參數X6、X7和X8對各項關鍵品質屬性和收率影響較大,為關鍵製程參數(Critical Process Parameter, CPP),必須對參數範圍進行嚴格的控制。其他參數對各項關鍵品質屬性和收率影響不大,製程參數的變動引起CQA超出範圍的風險低,故均為非關鍵製程參數(non CPP)。利用JMP軟體的確定性篩選實驗方法,只通過1輪24次實驗就成功研究了陽離子交換層析的製程特性,篩選出關鍵製程參數,制定了針對性的控制策略,確保最終產品安全可靠。如果對JMP 確定性篩選DSD感興趣的話,歡迎下載JMP30天免費試用。
- © 2025 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- Contact Us

You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.