Getting insight from data as quickly as possible for Pharma and Biotech
 HadleyMyers
HadleyMyers
 HadleyMyers
HadleyMyers

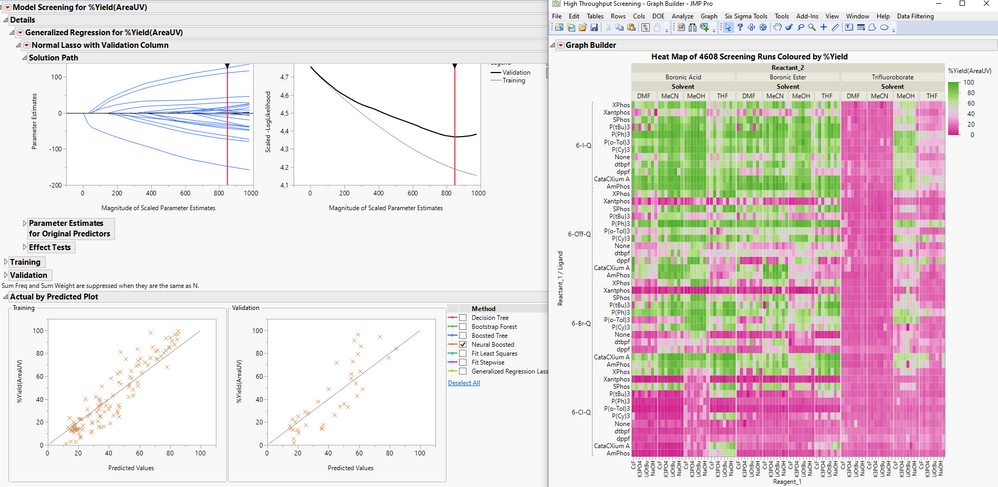
Here is a great presentation by @Phil_Kay using the new Model Screening platform in JMP Pro 16.
 HadleyMyers
HadleyMyers
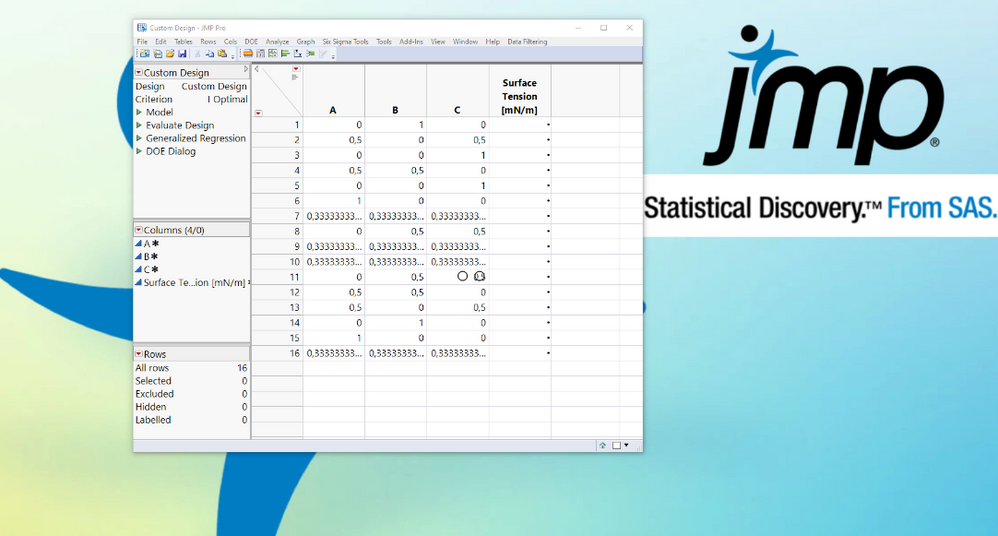
An analysis of a designed experiment in which the response is a curve measured over time. Scientists have defined an ideal curve shape and are searching for the optimal setting of a mixture that best approximates that shape.
 HadleyMyers
HadleyMyers
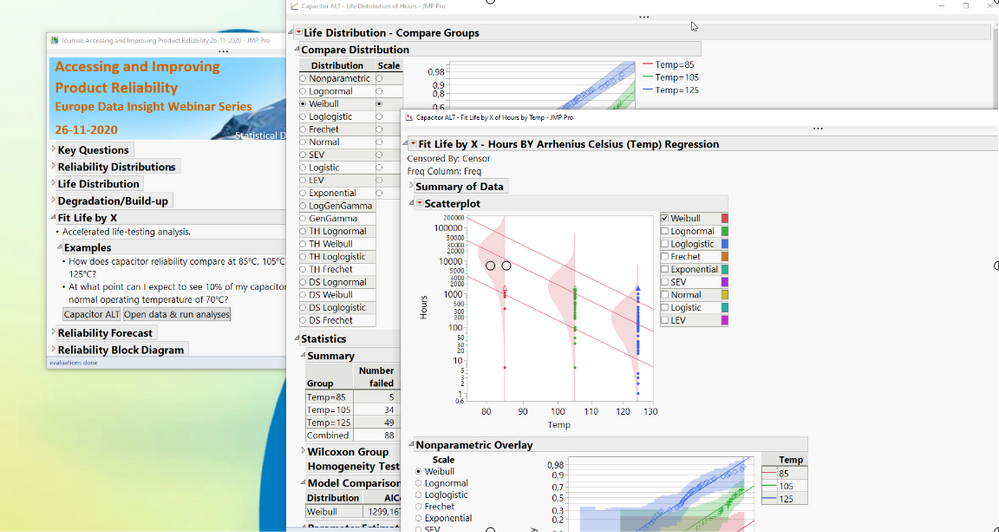
Using some of the platforms available in the "Reliability and Survive" menu to answer a few key questions, such as: How can we estimate the rate at which a product fails?How can we predict failure rates under different conditions?How can we determine warranty risks?How can we model failure in complex systems?
 HadleyMyers
HadleyMyers
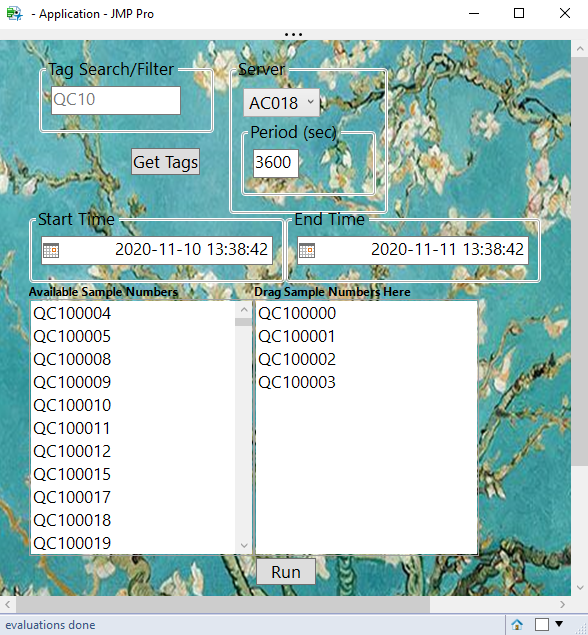
How to build custom applications to allow users to pull data into JMP using SQL or WebAPI.
 HadleyMyers
HadleyMyers
We can’t rely on error codes to correctly diagnose equipment problems. Doing nothing is never an option, and whole units might be changed or replaced at great cost rather than identifying a specific component responsible. Faults can also reoccur. An intermittent fault affecting a different unit or part of the equipment takes several failures before the true cause is diagnosed and identified. B...
 HadleyMyers
HadleyMyers

Hello everyone, The "Solving Problems through Data Visualization and Statistical Analysis" workshop was held on 9 June 2020 and repeated on 11 and 12 June 2020. In case you missed it, would like to see it again, or perhaps know a friend or colleague who might benefit, a recording of the event can be found below. Note that the workshop was meant as a "hands-on" virtual event for you to be able...
 HadleyMyers
HadleyMyers

JMP Benelux hosted a virtual workshop on Design of Experiments (DOE), which was repeated on different dates and times between 24-30 April 2020. The workshop focused on the benefits of modern Definitive Screening Designs (DSD), but also covered Mixture designs and cases with hard-to-change variables, two situations where DSDs can’t be used. The advantages gained by organizations who adopt an an...
 HadleyMyers
HadleyMyers
