現在の最新バージョン「JMP 17」には、医薬品の安定性試験分析に関する新機能が追加されました。具体的には、切片および傾きが異なるモデルで、誤差分散をプール(併合)して計算するオプションが導入されています。
このオプションを利用すると、切片および傾きが異なるモデルを選択した場合に、下図のような「誤差分散をプールして計算した」というメッセージが表示されます。そして、この計算方法に基づいて有効期間が算出されます。
特に、日本のJMPユーザーの中で、安定性試験の分析を行っている方々にとって、この機能は非常に便利です。しかし、このオプションが十分に知られていない可能性も考えられるため、本ブログでは具体的な例を交えながらこのオプションの意味と利用方法を詳しく解説します。
ICH Q1Eガイドラインの記述に準拠
端的に言うと、このオプションはICH Q1E(安定性データの評価に関するガイドライン)の記述にJMPを適合させるものです。
安定性試験では複数ロット(3ロット以上)のデータに対して共分散分析をおこなうことにより、以下のモデル1、モデル2、モデル3に分類されます。(モデル1、モデル2、モデル3は、JMPのレポートで出力される用語に合わせたものです。)
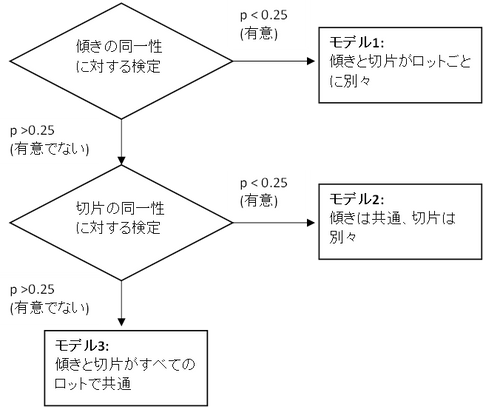
今回の焦点となるのは、傾きと切片がロットごとに別々のモデルであるモデル1に分類されたときのケースです。
このモデルに関して、ICH Q1Eガイドラインには、次のような記述があります。
傾きが等しいとする仮説が検定で棄却された場合(すなわち、ロット間に傾きの有意差が存在する場合)、全ロットのデータを一括することは不適切とみなされる。安定性試験を行っている個々のロットのリテスト期間又は有効期間は、個々の縦軸切片及び個々の傾き、並びにすべてのロットから計算した平均二乗誤差を用いて、B.1 項に記載された方法を適用して推定することができる。全ロットのリテスト期間又は有効期間として、個々のロットの推定値のうち最も短かいものを選ぶ。
上記の赤字で示した箇所が、誤差分散をプールすることに相当します。
先に示したオプションをチェックしない場合は、STABマクロというものの仕様に合わせロットごとに求めた誤差平方和を用います。JMP 16まではこの仕様でしか計算できなかったのですが、JMP 17では、先に示したオプションを利用することにより誤差分散をプールして分析できるようになりました。すなわち、ICH Q1Eのガイドラインの記述に合わせた分析ができるようになったのです。
新しいオプションの利用
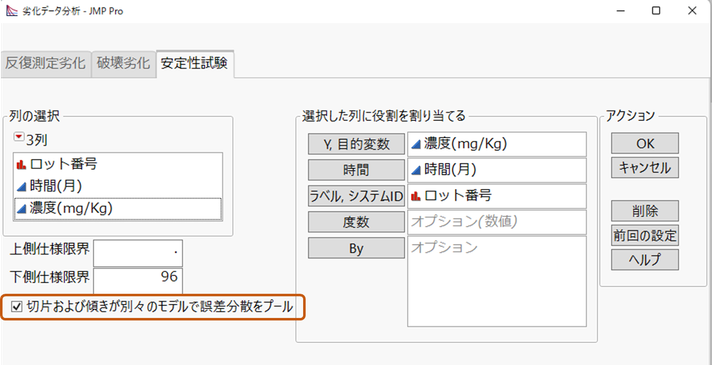
医薬品の安定性試験の分析を行うには、JMPのメニューバーから [分析] > [信頼性/生存時間分析] > [劣化分析] を選択し、列の選択ダイアログで左上のタブから「安定性試験」を選択します。
左下にある「切片および傾きが別々のモデルで誤差分散をプール」が新しいオプションです。デフォルトではチェックが入っていませんが、JMPのレポートを使って医薬品の承認申請を考えているのであれば、このオプションはチェックを入れておいた方で良いでしょう。
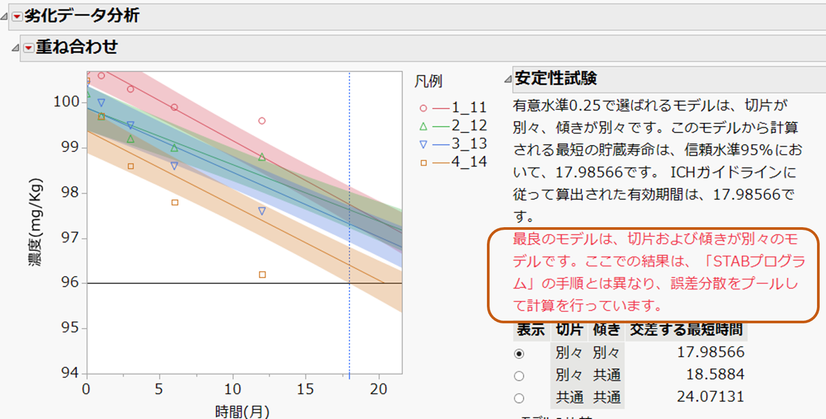
劣化データ分析のレポートが表示されます。この例では、上記のフローに基づくとモデル1(切片別々、傾き別々)が選択されており、右側に赤字で示しているように誤差分散をプールして計算した方法を示しています。
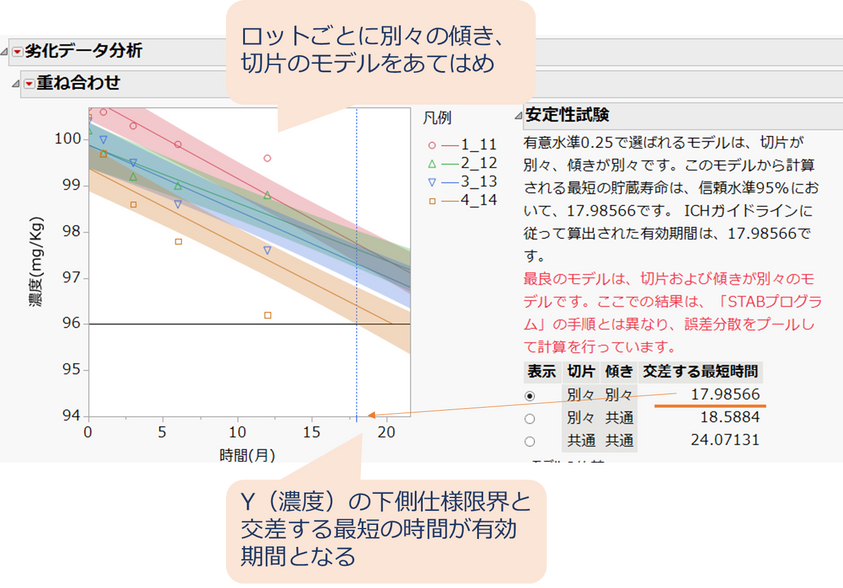
この例では、濃度の下側仕様限界を96と指定しています。そのため、Y軸である濃度=96と交差する最短の時間(17.986)が有効期間として示されます。
「モデルのあてはめ」でも有効期間を算出してみる
「劣化分析」では自動的にフローに基づいたモデルを選択し、そのモデルに対する有効期間を示すことができるのが特徴ではありますが、今回紹介した新しいオプションを使って有効期間を算出する手順を「モデルのあてはめ」を使って実施してみます。
1. [分析] > [モデルのあてはめ] を選択し、「Y」と「モデル効果の構成」を以下のように指定します。
ロット番号*時間(月)は、ロット番号と時間(月)の交互作用効果を示します。この効果を含めることによって、傾き別々、切片を別々に推定する回帰モデルを当てはめていることになります。
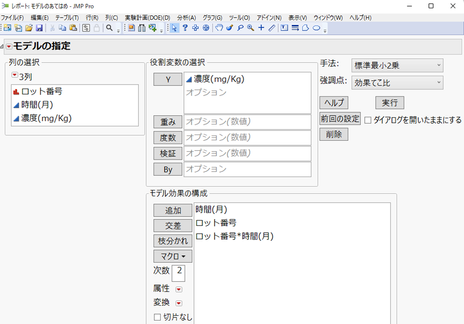
モデルのあてはめのレポートが表示されます。「回帰プロット」をみると、各ロットに対し傾きと切片が別々の直線があてはめられていることが確認できます。
2. レポート左上の赤い三角ボタンから [推定値] > [逆推定] を選択します。 ここで、(予測対象)の「すべて」にチェックを、「下片側」に変更し、濃度の下側仕様限界である"96" を入力します。
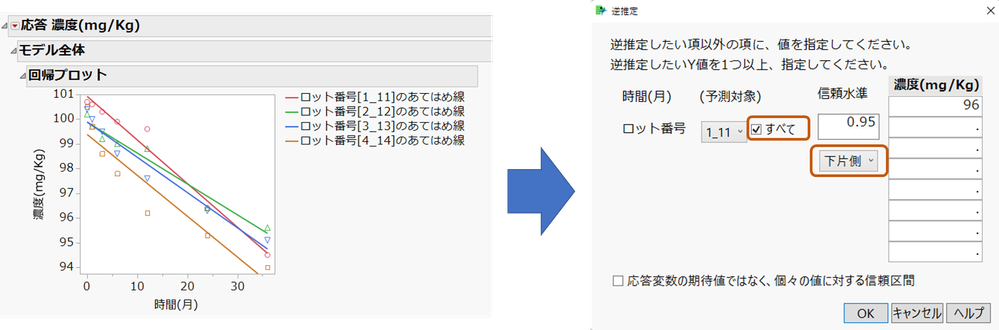
逆推定のレポートでは、ロット番号ごと逆推定における時間(月)の予測値、下側95%が表示されますが、ロットの中で下側95%の値が最も小さい値が有効期間となります。
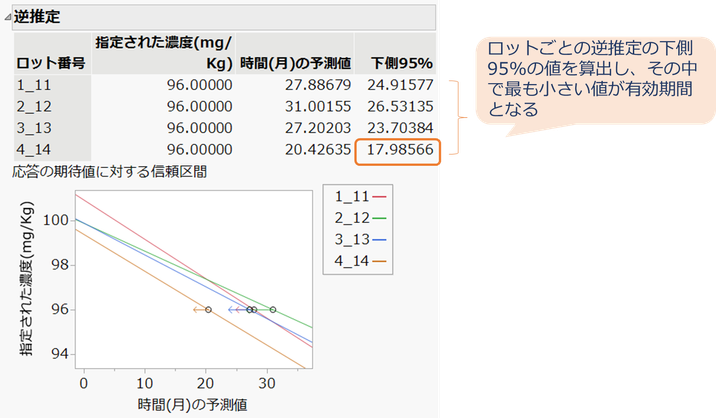
この例においてロット番号(4_14)の下側95%は17.986であり、劣化分析の安定性試験で算出された値と一致します。
安定性試験データの分析に対する技術資料(日本語)
JMPジャパン事業部では、JMPの安定性試験の機能について技術資料としてまとめたものを提供しております。
本ブログで示したことも含め、複数の包装形態があったときのモデル選択、アレニウスモデルを使った加速試験などの解説もありますので、興味のある方は是非ご参照ください。
JMPによる安定性試験データの分析 ~医薬品の有効期間設定のために~ (PDF版)お申し込み | JMP
by 増川 直裕(JMP Japan)
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.