- JMP User Community
- :
- Blogs
- :
- JMPer Cable
- :
- New features in JMP® Clinical 18
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
JMP Clinical 18 (planned for release March 2024) has several new and important features, including new reports, a new method for querying the study data, and a new clinical data mapping tool. In addition, a thorough review and overhaul of product performance provides greater speed for loading and analyzing studies.
JMP Clinical is a focused and specialized product for clinical trial data review. We give users “straight out-of-the-box” functionality to do thorough reviews of clinical trials at the study, site, and subject level. It is utilized across the pharmaceutical industry by drug sponsors and regulatory agencies.
JMP Clinical can be used for any type of clinical trial data analysis, and it comes with several built-in reports for clinical safety reviews, medical monitoring, and study monitoring. JMP Clinical 18 enhances this functionality with new capabilities described below.
New reports in JMP® Clinical 18
Drug-Induced Liver Injury report
The Drug-Induced Liver Injury (DILI) screening report is a comprehensive review of all the clinical trial data related to DILI. DILI is one of the most serious adverse outcomes for a drug product. Identifying subjects in a trial that have evidence of liver injury and understanding if it is related to exposure to the drug product are critically important in the review of a clinical trial.
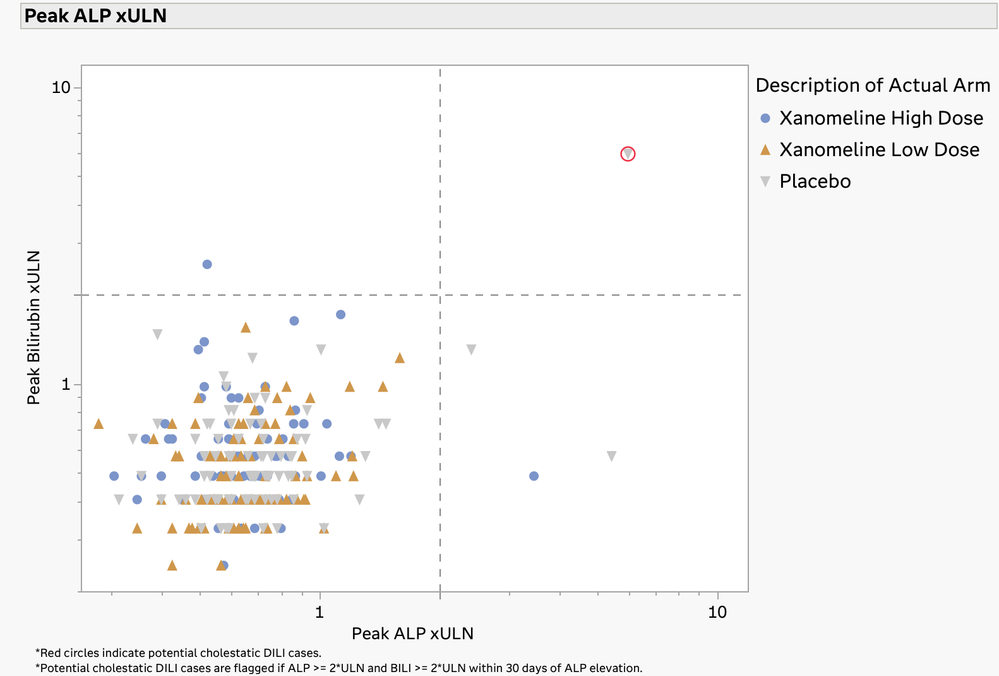
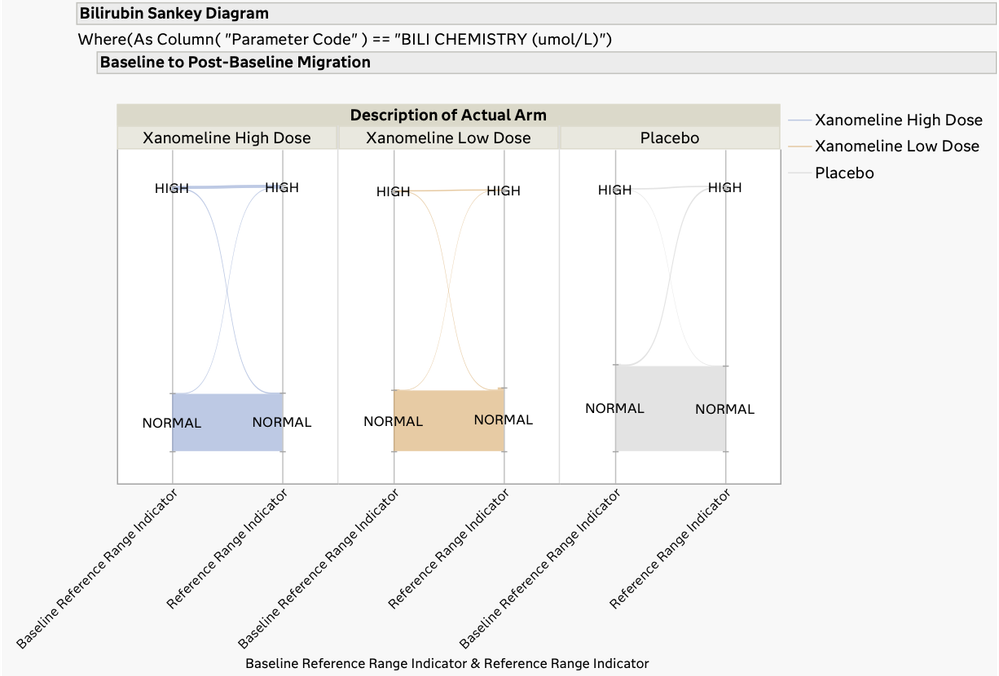
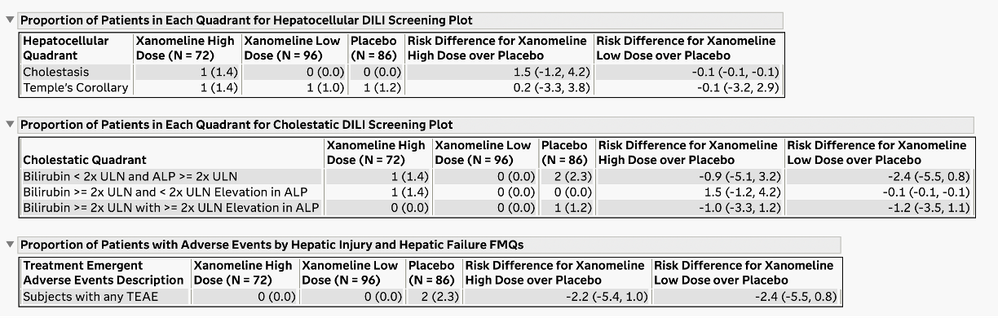
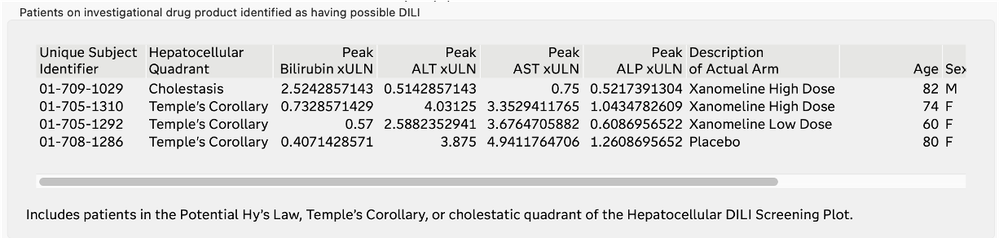
The U.S. FDA has published guidelines on the evaluation of DILI and has also provided draft guidance on several types of reporting examples that can be used for this screening. The DILI report in JMP Clinical 18 summarizes and combines information across several clinical data domains, including study visits, reported adverse events, laboratory findings, and subject disposition. These summaries are both visual and tabular. Ultimately, this report reveals the subjects in the trial that have possible DILI. All of the analysis and plots in this report have been steered by FDA guidance and JMP's long-standing interactions with that agency.
Hy’s Law Screening Plot
Migration of Patients from Baseline to Post-Baseline Lab Levels
Summary Tables for Medical Queries and Hy’s Law
List of Subjects with Possible DILI
Risk-Based Monitoring report
- Every clinical trial requires structured and detailed monitoring of the trial to ensure that the trial is being conducted as planned and that there are no serious execution or data errors occurring during the trial.
- Evidence of the health of the trial can be summarized in the risk metrics or risk factors that indicate how well the study is being conducted. These include monitoring for evidence of:
- Under- or over-reporting or adverse events or other safety related events.
- Frequency of protocol deviations or investigations.
- Rate of patient enrollment and dropout.
- Summaries are typically aggregated at the study site level, but you may also want to look at other groupings such as which study monitors are performing the site reviews and which organizations/vendors are monitoring the sites.
- Thresholds for the risk metrics are defined to give a risk rating of Low, Medium, or High that can then be used to make decisons about the trial monitoring process.
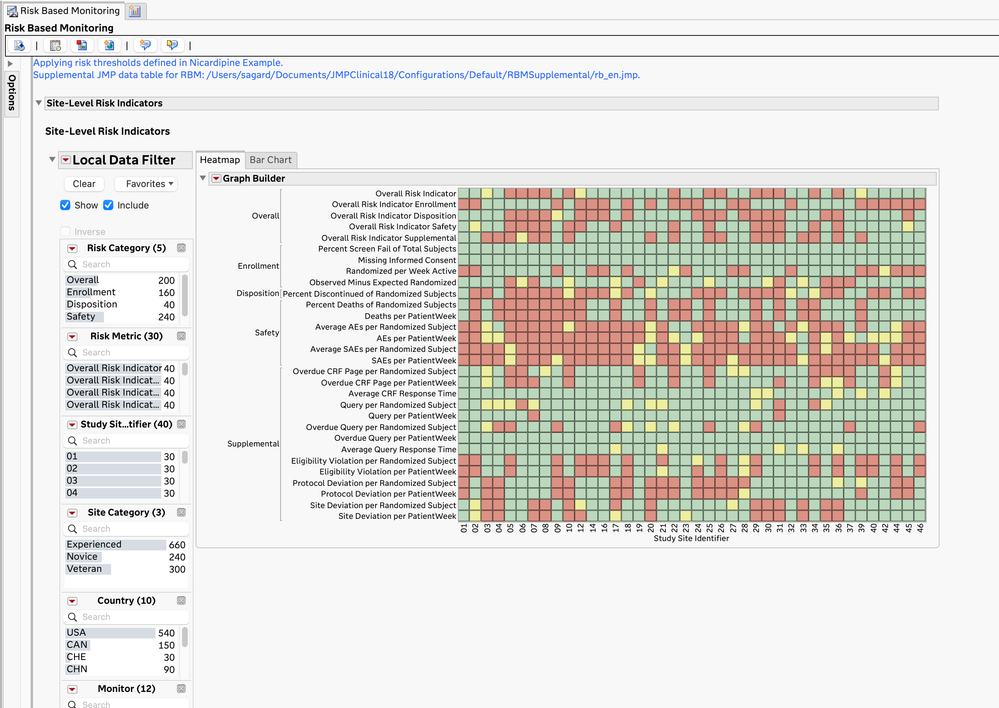
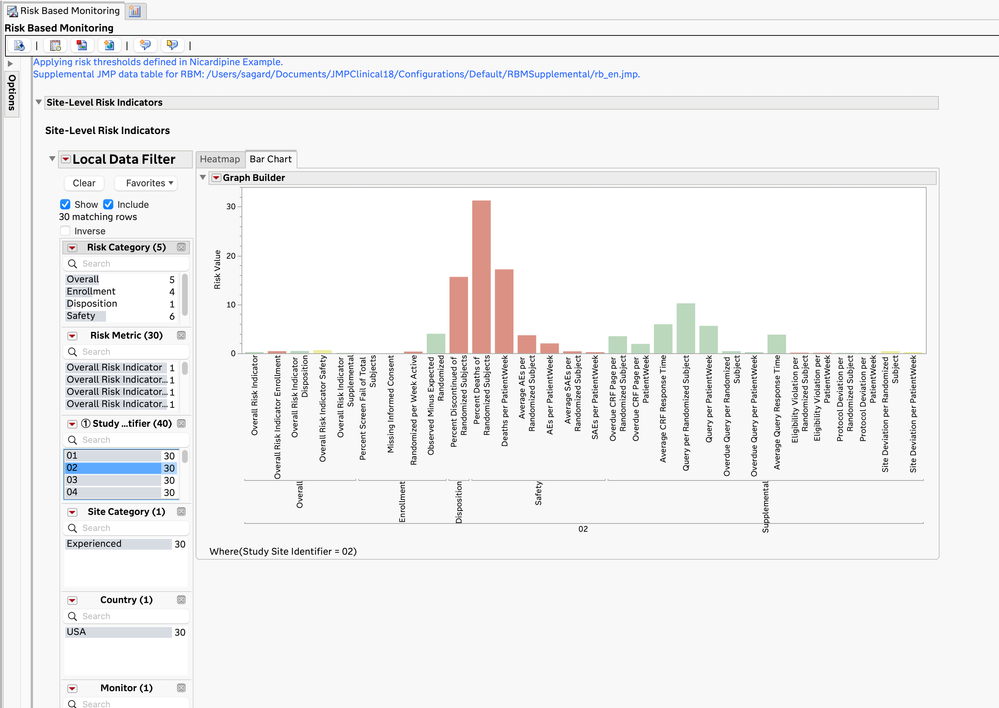
- The JMP Clinical Risk-Based Monitoring report shows a high-level, filterable summary of the risk metrics by site (and optionally, by monitor and vendor) and allows for closer examination of the risk metrics at each site.
Site-Level Risk Value Heatmap
Risk Values for Specific Site
A new way to query your clinical data: Subpopulation Builder
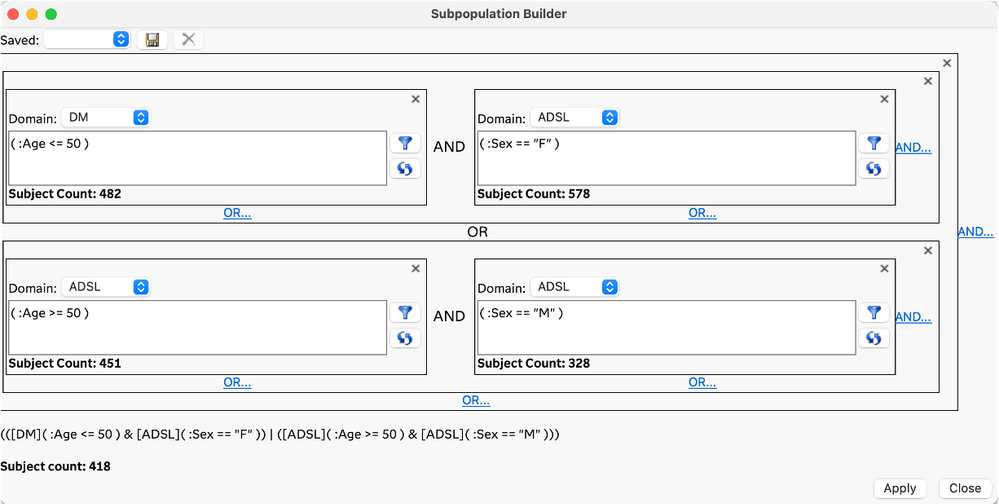
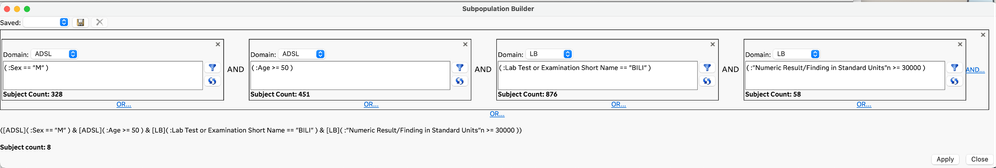
The planned and exploratory analysis that is typically performed on clinical trial data often requires identifying and/or filtering to a subset of subjects that meet conditions that can be defined by the data. Traditional approaches to developing complex rules for selecting subsets of subjects have required code-based conditional statements or queries. The Subpopulation Builder is a new GUI-based approach in JMP Clinical 18 that allows you to specify complex queries across all the clinical data domains so that you can filter to the subjects of interest. This new approach is easy to use and does not require coding.
Subpopulation Builder addresses our users’ desire to easily use complex cross-domain queries that are important to them and that are focused the type of analysis they want to do. These subpopulation queries can also be modified manually using JMP Scripting Language when an even more complex query is needed. Finally, these subpopulation queries can be saved and applied as filters directly to the study reviews in progress, automatically updating the analysis to focus on just those subjects of interest.
Query: All females under age 50 and all males 50 and older
All males 50 and older with a bilirubin lab result >= 30000
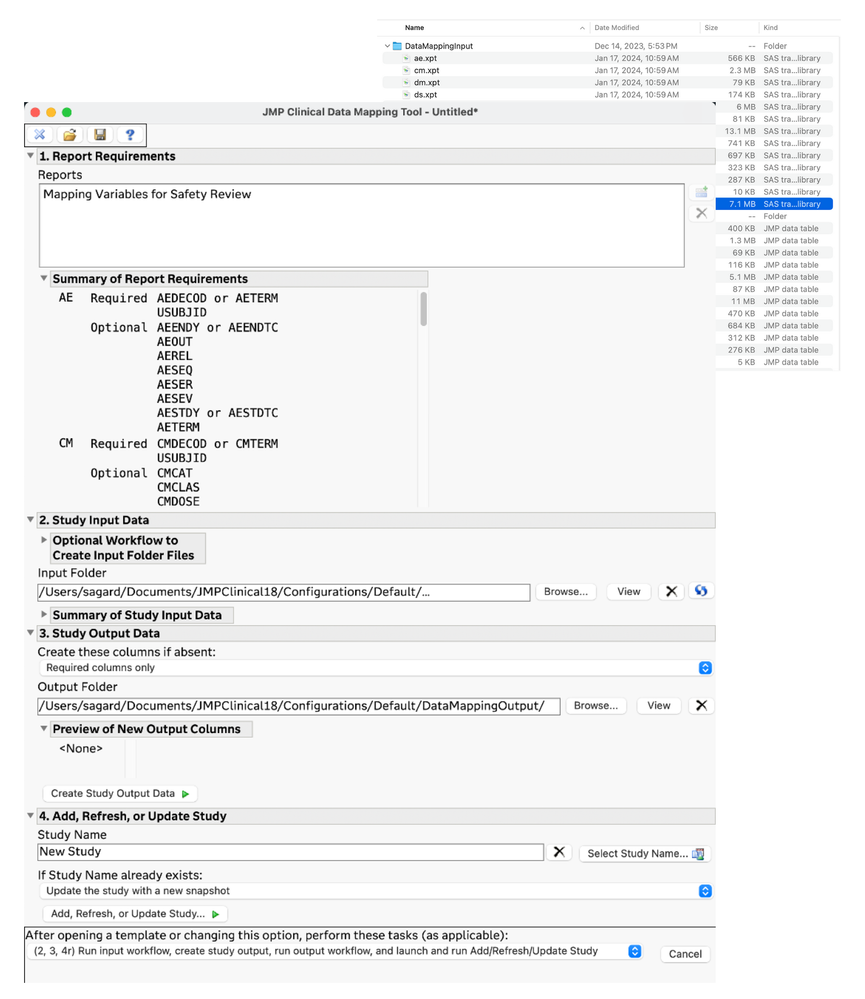
New Clinical Data Mapping tool
The Clinical Data Mapping (CDM) tool is an add-in for JMP Clinical that allows you to go from raw clinical data to transform, map, and load that data as a study in JMP Clinical.
Many organizations and reviewers lack resources or systems to provide them with CDISC standard SDTM data files as input for clinical data analysis tools. This new tool facilitates that process and enables users to prepare clinical data – in a repeatable way – so that it can be loaded into JMP Clinical for study review.
The starting data can be in any form that JMP can read in, including text basedfiles, Excel workbooks, SAS data sets, and JMP data tables.
A minimum list of required variables is displayed, and you can import, transform, and/or map your data to the required variables. Pre-processing is facilitated by allowing the use of JMP workflows from the Workflow Builder, and post-processing the prepared data can also be done with a workflow. The data mapping can be driven manually, step-by-step, until you have your data ready for input, and then you can pass that prepared data to the Import Study tool in JMP Clinical. All of this work can be saved so that you can document the process that you used to get your data ready. It is also reusable so that you can also reapply the same steps to data when you get new or updated data.
- © 2024 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- About JMP
- JMP Software
- JMP User Community
- Contact

You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.