Recently, a JMP colleague asked me if there were any documents from the FDA discussing the usage of JMP or JMP Clinical for reviewing clinical trials submissions. I replied that, at PHUSE and DIA conferences, FDA employees had mentioned that medical and statistical reviewers were piloting or using JMP and JMP Clinical; however, nothing was definitive about how widely our products have been used there, some since as early as the first version of JMP.
But I am a big believer in Google searches turning up gems, and this conversation led me to run searches on both Google and Google Scholar. Well, much to my surprise, something new showed up! But before I divulge my search results, let me provide a little background on medical doctors using JMP products over the years.
JMP has quite a few success stories about medical doctors working for hospitals, research universities, pharmaceutical companies and other life sciences organizations. The stories describe the value of our products to these individuals and organizations focused on dealing with patient data. You can read many of these at our Life Sciences Industry Success Stories website.
Recently, as the success stories show, we have gained ground with medical doctors by providing value for those involved with evaluating clinical trials data using JMP, JMP Pro, JMP Genomics and JMP Clinical. This is true for regulatory agencies, clinical research organizations, universities and biopharmaceutical companies developing or evaluating drugs and biologics for the treatment of diseases. However, one type of medical doctor -- a medical officer at a regulatory agency -- has no customer success story, as regulatory agencies are careful to maintain vendor neutrality. However, they often provide information at conferences and on their websites about their usage of particular software products.
United States Food and Drug Adminstration (FDA)
My first search result uncovered a document that is an update to the Manual of Policies and Procedures for the Center of Drug Evaluation and Research at the US FD. It's the CDER Manual of Policies and Procedures for Medical Reviewer Training, and it indicates that JMP and JMP Clinical have become valuable to medical reviewers. During months 9-18 of a medical officer's training at the FDA, the office of computational sciences provides required training on JMP and JMP Clinical. Here is a screenshot of page 8 of the manual:
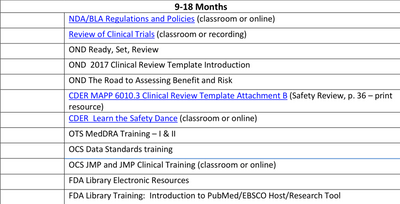
I was so excited to see this that I shared it with many of my colleagues because so many of us at JMP are committed to helping make data scientists out of medical doctors who would like to evaluate the results of clinical studies themselves. This document indicates to me that JMP and JMP Clinical have become essential to these individuals to complete their submission reviews. We are starting to see this behavior across multiple industries because JMP products provide so much value above and beyond what typical visualization software can provide.
Japan's Pharmaceutical and Medical Devices Agency (PMDA)
So I thought: Why stop there since I know that other regulatory agencies use JMP products for evaluating drug submissions? My PMDA search resulted in finding a presentation given last year by Dr. Yuki Ando at the Japanese Pharmaceuticals and Medical Devices Agency, showing how medical doctors and statisticians review clinical studies at their organization using JMP and JMP Clinical. You can view the presentation, titled Electronic Data Submission and Utilization in Japan, yourself. Here's a screenshot from the presentation if you don't have time to read her presentation.
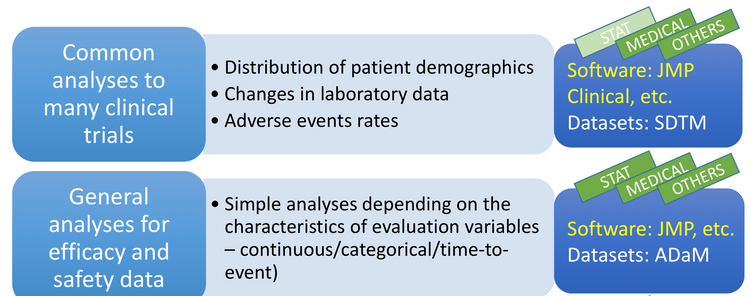
I have had the pleasure of meeting Dr. Yuki Ando at DIA and PHUSE conferences around the world as she keeps her organization at the forefront of regulatory agency policy and standards usage, as well as software and analytics for biopharmaceutical products. On a side note, in Japan regulatory agencies are very transparent about their purchase of software products, and the announcement of the purchase of JMP Clinical, in 2013, is available online, PMDA purchase of JMP Clinical (in Japanese), as is a translation of that page, PMDA purchase of JMP Clinical translated into English.
Other regulatory agencies
There are other regulatory agencies using JMP products. Hopefully, they will publish documents sharing their usage in the near future. So stay tuned for updates to this blog post. You can find other documents on the web that mention JMP and JMP Clinical use at regulatory agencies.
If you know of other public documents from regulatory agencies showing JMP, JMP Pro, JMP Clinical or JMP Genomics being used, please share them in the comments.
If you have not done so, please visit our website to learn how JMP products are changing the world.