김대윤의 블로그 - DaeYun Kim's Blog
- JMP User Community
- :
- Blogs
- :
- 김대윤의 블로그 - DaeYun Kim's Blog
- :
- 임상시험 정기적 안전성 정보보고(DSUR)?
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
FDA에서는 2011년 부터 가이드라인을 제정하여 약물감시(Pharmacovigilance)에 있어 DSUR(Development Safety Update Reporting) / PSUR(Periodic Safety Update Report) 보고를 의무화하는 규제를 시행 중이고, EMA 역시 2011년부터 PSUR 규제를 시행 중입니다. 아시아권도 다소 차이는 있지만, 일본과 중국은 이미 시행 중인데 반해 한국 식약처는 2021년부터 시행을 준비 중에 있습니다. 식약처에서는 2021. 5. 12일 임상시험용의약품 최신 안전성정보 보고(ICH DSUR) 관련 법령을 개정하였습니다. 식약처에 따르면 DSUR 제도는 임상시험용의약품에 대하여 문헌, 비임상·임상 자료 등 안전성 정보를 수집·평가해 정기적으로 보고하는 제도로, 2022년 의무적 시행을 목표로 현재 「약사법」 하위규정에 대한 개정을 추진 중입니다.
관련자료
뉴스기사: DSUR 의무화 임박…제약 "힘들지만 도입 필요성 공감"
임상시험용의약품 안전성정보 보고 내년부터 의무화(식약처, ICH 등 국제기준 반영해 DSUR 제도 의무 시행)
EMA: EMA PSUR
MFDS: [식품의약품안전처] 의약품 안전관리 2020년 시행계획
임상시험용의약품 최신 안전성정보 보고(ICH DSUR)(민원인안내서)- 2021. 5.12 제정
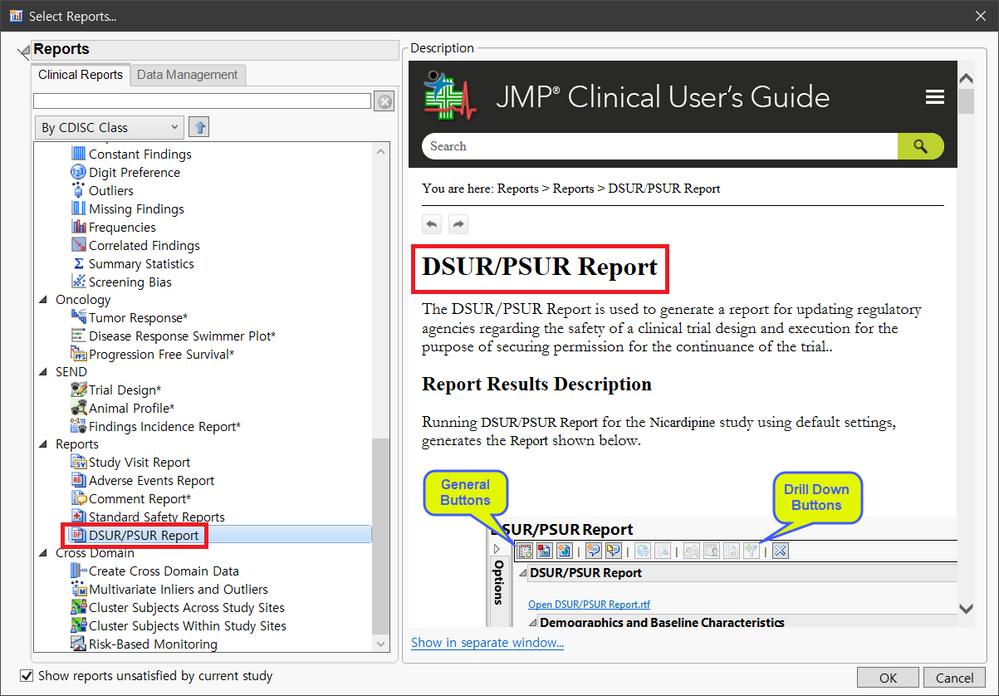
임상시험에 특화된 소프트웨어인 JMP Clinical 의 최신 버전은 8.0(작년 11월 업데이트)으로 FDA, EMA, PMDA 등 여러 규제기관의 새로운 지침과 Reviewer들의 의견을 반영하여 지속적인 버전 업데이트를 진행 중에 있습니다. 특히 이번 8.0버전에서는 규제기관에 제출할 수 있는 최대 5개의 테이블과 3개의 목록(아래 그림 참조)을 빠르고 효율적으로 생성할 수 있는 DSUR/PSUR 보고 기능이 추가되었습니다.
JMP Clinical에서의 DSUR/PSUR 관련 상세 기능은 아래 링크를 통해 확인하실 수 있습니다.
DSUR/PSUR report in JMP Clinical: Assess safety in ongoing clinical trials
참고: JMP Clinical 8.0의 새로운 추가 기능
1. Adverse Event Narrative Summary
2. DSUR / PSUR Report
3. Medical Query Risk Report
4. JMP Live와의 연동
JMP Clinical 관련 문의 : daeyun.kim@jmp.com
감사합니다.
- © 2026 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- Contact Us


You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.