- We’re retiring the File Exchange at the end of this year. The JMP Marketplace is now your destination for add-ins and extensions.
- JMP 19 is here! Learn more about the new features.
- Subscribe to RSS Feed
- Mark Topic as New
- Mark Topic as Read
- Float this Topic for Current User
- Bookmark
- Subscribe
- Mute
- Printer Friendly Page
United Kingdom JMP Users Group Discussions
- JMP User Community
- :
- JMP Users Groups
- :
- JMP Users Groups in EMEA
- :
- United Kingdom JMP Users Group
- :
- Forum
- :
- Get ready for some impressions from the UK JMP Users Group on July 9
- Mark as New
- Bookmark
- Subscribe
- Mute
- Subscribe to RSS Feed
- Get Direct Link
- Report Inappropriate Content
Get ready for some impressions from the UK JMP Users Group on July 9
Hi all,
This years UK Users Group Meeting was originally planned to be a physical meeting that should have been held in Liverpool at the MIF. Coping with a new situation the UK steering committee turned it into a virtual event because for many people it was not possible to travel. We would like to thank the presenters for their great contributions as well as the many loyal and new users who attended last week!

I am uploading the presentation slides and some impressions of the meeting here
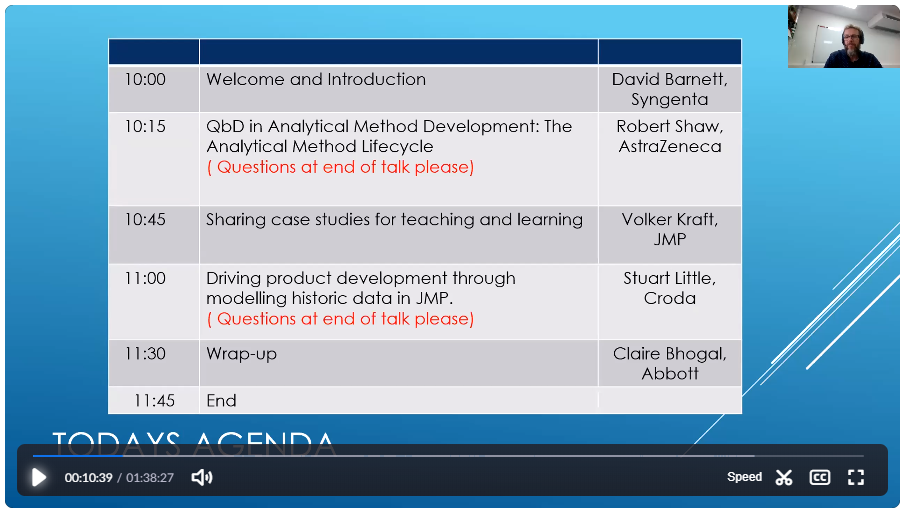
|
10:00 |
Welcome and Introduction, David Barnett, Syngenta
|
|
10:15 |
QbD in Analytical Method Development: The Analytical Method Lifecycle
|
|
10:45 |
Sharing case studies for teaching and learning

|
|
11:00 |
Driving product development through modelling historic data in JMP
|
|
11:30 |
Wrap-up, Claire Boghal 
|
|
11:45 |
End |
- © 2025 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- Contact Us