A pharmaceutical company was losing money and credibility due to a quality issue with a tableting process. In this case study you will see how they found a robust solution to fix the problem for good, increased their credibility with externals and saved many $100k per year in scrapped batches.
This case study shows how you can solve scientific and engineering problems by exploring data with the interactive visual and statistical tools in JMP.
This is a schematic of the multi-stage process for producing tablets from the Active Pharmaceutical Ingredient: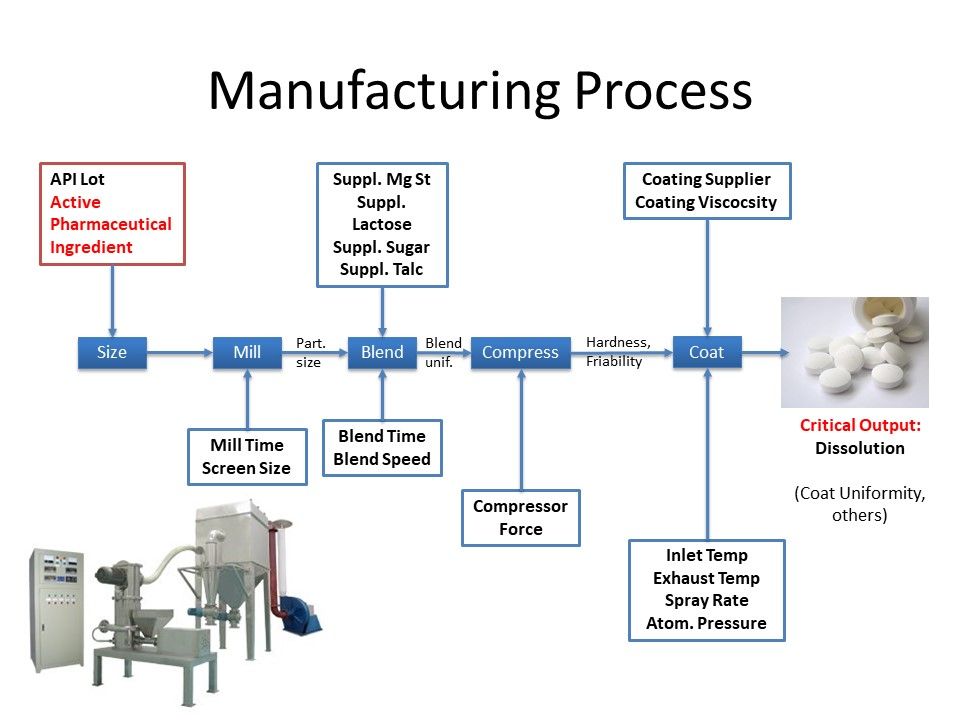
What were the problems they faced?
- Scrapping 15% of batches due to non-conformance to release specification.
- The process owners breathed a sign of relief whenever a good batch was released.
- QA would investigate failing batches but conclude there was no assignable cause.
- This pattern continued over many years.
- Then, after an audit, they received a letter warning that license to manufcature could be removed.
- This threat had serious financial consequences as the product was off-patent and still generated significant revenue.
How did they solve the problem?
By visually exploring the process data for clues to causes of out-of-spec output they gained a rich understanding of the process.
Using this approach they were able to find a robust solution to fix the problem for good. This increased credibility with externals and ultimately saved many $100k per year in scrapped batches.
They identified that just a handful of the process variables were important drivers of variation. A statistical model showed them how to control the process to consistently produce quality product:
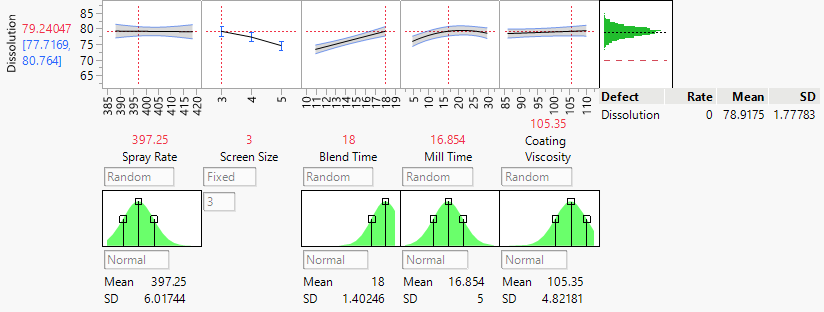
Find out more about improving your processes with visual and statistical exploration in JMP.
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.