In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Clinical Reviews) templates to show how drug safety can be evaluated. My previous blogs demonstrated how to capture SAEs using Adverse Events Narrative3, and Discontinuations Due4 to AEs and TEAE5 with Adverse Events Distribution. There is a final step in Safety Results evaluation: determining if there were any significant adverse reactions.
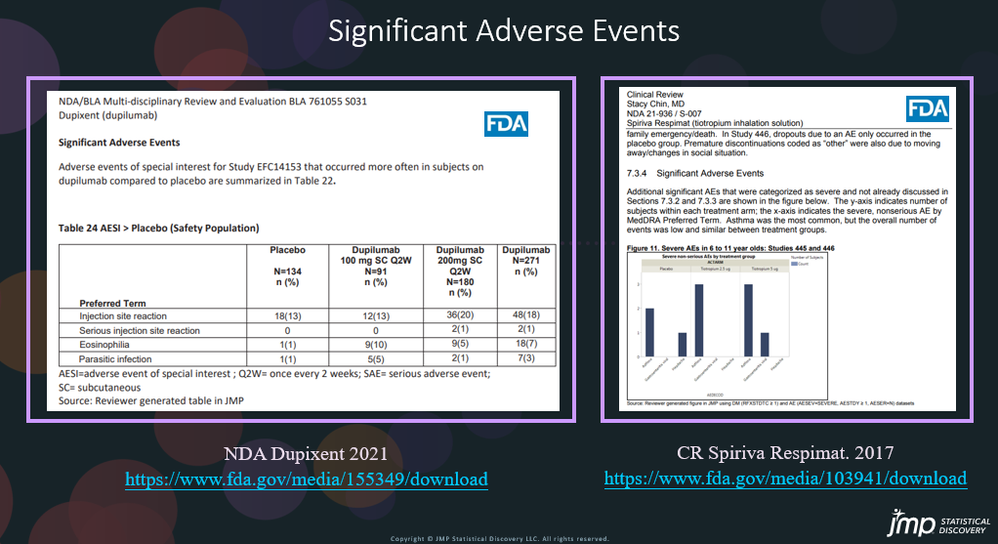
Significant different adverse events between the treatment and placebo group need to be identified. In the NDA for Dypixent6 and CR for Spiriva Respimat7, JMP was used to create a table or bar chart to illustrate any differences between the groups for certain adverse events.
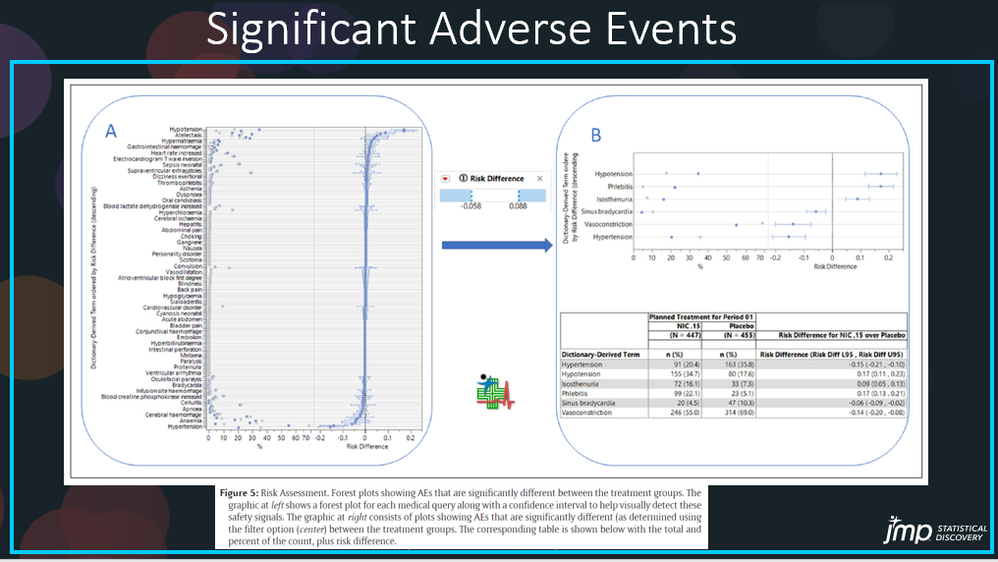
JMP Clinical offers another way to present the significant AEs. The popular forest plot is generated by Adverse Events Risk Report to assess the risk and identify significant different AEs between the treatment groups as shown in Figure 5 in the paper1. The overall risk of AEs (Figure 5A) can be filtered by Risk Difference or Absolute Risk Difference to reveal significantly different AEs between the treatment groups, as shown in Figure 5B, along with the corresponding table, shown below, that lists the total counts and percentages. The Medical Query Risk Report can be used to further look at these safety signals using adverse events and corresponding medical queries, such as FDA Medical Query and Standard Medical Query, which will be explained in my next blog.
References
- Mann, G. & Pedersen, T. J. & Lyzinski, R. & Scott, A. & Foglia, A. J. & Cromer, J. & Dong, M. & Varga, N. & Gardner, S. & Kirchberg, C. J. & Wingerd, B. A. & Wolfinger, R. D. & Bao, W., (2023) “CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation”, Journal of the Society for Clinical Data Management 3(1). https://doi.org/10.47912/jscdm.169
- CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation published in JSCDM
- Safety Results I: Death and SAE described by AE Narrative in JMP Clinical
- Safety Results II: Discontinuations Due to AEs Evaluated by AE Distribution in JMP Clinical
- Safety Results III: Treatment Emergent Adverse Events by AE Distribution in JMP Clinical
- NDA Dupixent 2021 https://www.fda.gov/media/155349/download
- CR Spiriva Respimat. 2017 https://www.fda.gov/media/103941/download
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.