Process analytical technology (PAT) is not new to the world, but it may be a newer concept for many, especially when it comes to using JMP to analyze and process PAT data. PAT can be part of and is often combined with Quality by Design (QbD).
PAT has been around since at least the late '90s and maybe even before. According to Microsoft Copilot, the use of PAT is endorsed by the U.S. FDA "as regulatory framework that encourages continuous improvement in pharmaceutical development, manufacturing, and quality control processes. By focusing on critical process parameters (CPPs) and critical quality attributes (CQAs), PAT aims to enhance product quality, consistency, and efficiency. Implementing PAT can lead to better products for patients." That said, CPPs and CQAs are important in any process, and there is no reason PAT cannot be implemented in any industry.
The main focus of PAT is to improve product consistency for quality, to help reduce waste, and to minimize rejects. The biggest challenges with PAT are knowing how to start collecting, and then analyzing, your data. Luckily JMP offers several options for pulling your data in for cleanup and analysis including functional data analysis (FDA). When combined with JMP Live, you have the complete package for monitoring, communicating, and updating process analyses.
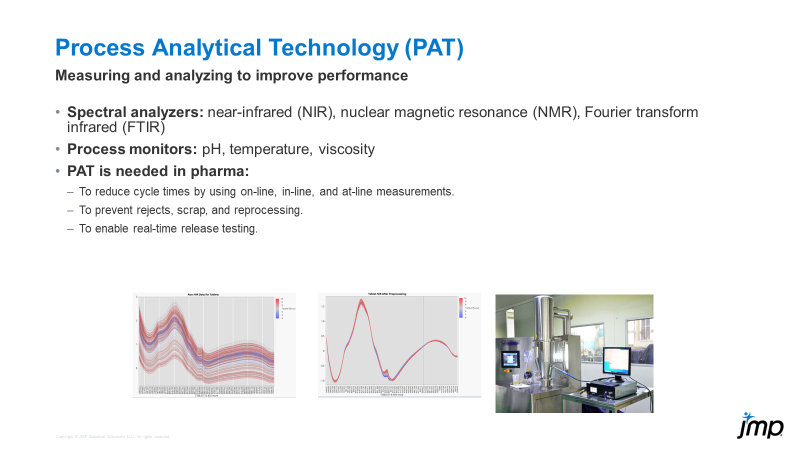
The presentation slide below gives an idea of how near-infrared (NIR) data can be collected and analyzed as part of a PAT application.
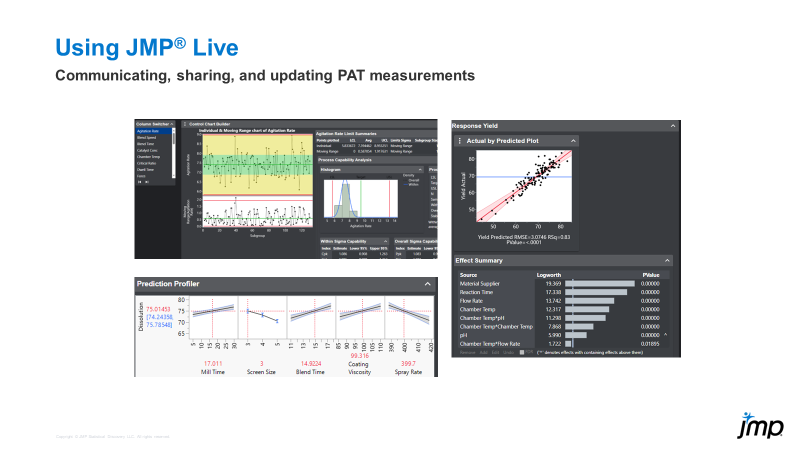
The slide below shows some of the capabilities of JMP Live for sharing and communicating analyses results. The whole process can ultimately be automated, including the data update step(s).
Keep JMP in mind when you are looking at implementing PAT. If you have any examples, please share them via blog or at an upcoming Discovery Summit in your region. We look forward to hearing about your PAT success stories with JMP!
Check out Massimo and Sara's poster presentation at the link below. I have attached a slide show outlining the application of PAT that was produced along with my colleague @PIN - Pin Hu.
Consolidation and Integration of Data Workflows to Guarantee Manufacturing Proce... - JMP User Commu...
Pharma Continuous Manufactig (PCM)_Bill Worley_Pin_v2.pptx
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.