在現代製造業中,穩定的品質控制對企業的競爭力至關重要。隨著製造程序日漸複雜,僅靠傳統方法已無法有效識別隱藏的品質問題。為解決這一挑戰,先進的資料分析工具成為品質工程師的利器。
本篇文章將實際探討一個產業案例—如何透過分析方法和工具解決複雜的品質問題,涵蓋從初步資料探索到最終決策的全過程。
問題背景
某製造企業在生產製造過程中發現,兩種關鍵原料(化合物1與化合物2)的品質波動較大,影響了產品的一致性與可靠度。初步分析顯示,這種波動可能與原料供應商的生產製程差異有關。如何找出品質波動的根源,並制定相應的控制策略,是當前的核心問題。
為深入理解問題,該團隊決定對資料進行多角度分析,包括以下4大層面:
- 控制圖監控原料品質:評估是否存在特殊原因引發的波動。
- 供應商對比分析:識別不同供應商的制程差異及其影響。
- 過程能力評估:測量生產制程的穩定性和適配性。
- 多元統計分析:在多變數之間尋找潛在關聯。
分析前檢查常態分佈
團隊在分析資料前,意識到許多品質工具假設資料來源於常態分佈。如果不符合此假設,則基於平均值和標準差的結果將不具意義。因此團隊透過分佈分析確認化合物1和化合物2資料均符合常態分佈。

圖一:檢查常態性
控制圖與製程穩定性

圖二 : 控制圖
團隊使用控制圖工具進一步分析品質問題。控制圖透過上限(UCL)、中心線和下限(LCL)劃定範圍,用於區分普通變異和特殊變異。通常需要採取行動消除特殊變異,同時量化普通變異以確定製程能力。
在控制圖中:
- 製程資料生成的點如果隨機分佈在控制限內,表明製程穩定(僅存在普通變異)。
- 若點落在控制限外,說明存在特殊變異。
結果顯示:
- XBar圖中所有點均在控制限內,且隨機分佈,表示製程穩定。
- R圖中批次10超出控制限,顯示出特殊變異。
製程能力指標
Cp衡量規格範圍與製程分佈寬度的比值,而Cpk進一步考慮了製程中心相對於規格限的位置。通常要求Cpk ≥ 1.33為最低標準。
- 化合物 1 的控制圖:所有點都落在管制界限內,點的分佈隨機,表明流程受控且穩定。但 R 圖顯示批次 10 超出管制界限,表明存在一些特殊原因變異。Cpk 值為 0.740,Cp 值為 0.805,表明化合物 1 不穩定且組內極差較大無法滿足規格。
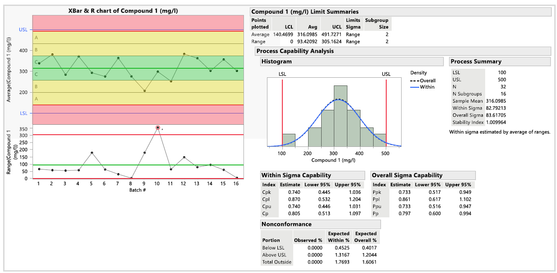
圖三 : 化合物 1 的控制圖及製程能力指標
- 化合物 2 的控制圖:XBar 圖顯示批次 11 落在紅色區域,表明流程不受控且不穩定。R 圖顯示批次 5 超出管制界限,表明存在一些特殊原因變異。Cpk 值為 1.005,Cp 值為 1.154,表明化合物 2 不穩定且無法滿足規格。
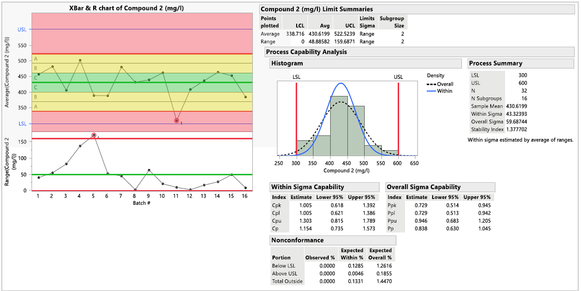
圖四 : 化合物2的控制圖及製程能力指標
供應商分析
在發現兩種化合物存在特殊工序變異後,團隊將下一步集中在從供應商角度探索這些變異。將每個供應商的控制圖並排展示,有助於透過圖形直觀地理解變異來源。

圖 5:按供應商分組的化合物 1 和化合物 2 控制圖
從圖 5 中可以看出,結果顯示,供應商 A 的批次間變異比供應商 B 更大,但供應商 A 對於兩種化合物都是穩定的,而供應商 B 在化合物 1 中表現出特殊原因變異
模型驅動的多元控制圖 (MDMVCC)
使用模型驅動的多元控制圖可用於監控多個工序參數。這種控制圖基於主成分或偏最小二乘模型構建,用於檢測各維度獨立監控時可能忽略的多維不穩定性。此外,使用者可互動式深入分析單個變數對整體信號的貢獻。

圖 6:供應商 A 和 B 的模型驅動多元控制圖
從圖 6 可以看出,供應商 A 的 T² 值在控制範圍內,但供應商 B 在批次 9、10 和 11 中表現出明顯的不穩定性。
工序能力分析
工序能力分析評估工序相對於規格限制的表現。一條穩定的工序應始終在規格範圍內生產產品。

圖 7:供應商 A 的化合物 1 和化合物 2 工序能力細節報告

圖 8:供應商 B 的化合物 1 和化合物 2 工序能力細節報告

圖9: 供應商 A 的化合物 1 和化合物 2 Goal Plot和製程性能圖
圖10: 供應商 B 的化合物 1 和化合物 2 Goal Plot和製程性能圖
觀察結果:
- 供應商 A 的兩種化合物皆在規格內但製程能力不佳,且長期變異及短期變異無明顯差異。
- 供應商 B 的化合物 2 在規格內但製程能力稍嫌不足,而化合物 1 在規格內且製程能力較化合物 2佳,但似乎長期變異和短期變異有明顯不同。
分析後決策行動
在檢測到原料中的特殊原因變異後,團隊決定採取以下措施:
- 引入監控工具:建議供應商使用I&MR圖和多變數控制圖,持續監控原料品質和關鍵製程參數。
- 提高檢測頻率:增加原料品質分析頻率;為解決現有檢測方法過慢的問題,研發超高效液相色譜(UHPLC)方法,用於快速檢測並降低資料瓶頸。
- 優化測量流程:改善現有測量程式,降低高測量偏差帶來的誤差風險,為後續製程自動化奠定基礎。
- 目標導向改進:透過Goal plot評估整體製程穩定性和能力,確保所有改進措施都圍繞核心品質目標展開。
小結
品質工程師的目標是實現卓越製造。持續識別並控制工序變異源,是品質部門的重要職責。透過在工序中內嵌品質控制,不僅提高靈活性,還確保產品和服務的需求得以滿足。
特別說明一下,本案例應用了以下幾個JMP主要功能,幫你透過數據分析辨識不同材料的變異性,包含但不限於:
- Distribution 能直觀地展示資料分佈,以及多個品質與工序平臺,
- Control Chart Builder 可以幫助你直觀理解變異來源
- MDMVCC多元控制圖則可用於監控多個工序參數
- Process Capability 平臺分析了工序能力和穩定性,並利用Goal Plot和工序表現圖將變異與能力結合,適用於需要監控大量工序的場景。
如果想要透過科學的統計分析和工具應用,該案例為複雜品質問題提供了系統性解決方案。在整個過程中,統計程序控制(SPC)和製程能力評估(Process Capability Analyze)起到了關鍵作用,幫助團隊有效識別特殊原因波動並優化生產制程。
要實現卓越的製造品質,需要持續的製程優化和品質監控。透過構建穩定且具備高能力的生產流程,不僅能降低產品不合格率,還能顯著提升企業的市場競爭力。
如果您也在尋找適合自己的品質管理解決方案,不妨點擊以下連結獲得更多學習資源:
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.