JMP를 이용한 Data 분석 과정 및 결과를 공유하는 방법은 여러가지가 있습니다.
1. MS Powerpoint/Word/Excel 등의 Report로 Export 하거나 pdf 파일로 변환하는 방법
2. Interactive Html with Data를 이용한 Interactive한 결과 공유
3. 웹에서의 공유 및 협업을 위한 JMP Pubic 혹은 JMP Live 이용
4. (오늘의 주제) Data Table에 분석 과정 및 결과를 Script(JSL)로 저장하는 방법
JMP Software는 Data를 통한 Insight 발견 및 공유가 다른 Software 대비 월등한 프로그램입니다. 특히 제약/바이오 업계의 경우 규제기관(식품의약품안전처)에 Data를 제공하고, 분석결과(심지어 분석에 이용된 Program까지)를 제출해야 합니다.
만약 JMP를 활용한다면 Data 분석 과정과 결과 등에 대해서 어떻게 공유하고 협업할 수 있을까요?
JMP Software는 여러분이 하시는 모든 작업을 background에서 Script화 하고 있습니다. JSL(JMP Scripting Language)이라고 하는 고유의 언어를 통해 작업과정과 그 결과를 저장하고 있지요. 만약 여러분들이 주어진 Data를 통해 작업한 과정과 결과를 저장하고 싶으시다면 단순히 Red Triangle >> Save Script >> To Data Table 과정만 적용해 주시면 됩니다.
혹은 더 간단한 방법으로는 "단축키"를 만드셔서 저장하실 수도 있습니다. 관련된 내용은 다음 링크(스크립트를 JMP 데이터 테이블에 저장하는 단축키 만들기)를 참고하세요.
아래의 Sample Data(JMP에서 기본적으로 제공되는 Sample Data입니다)는 Tablet을 만드는 제조과정에서 QbD를 적용하여 CQA, QTPP 등을 찾아나가는 과정을 직접 따라하실 수 있습니다. 아래 굵고 빨간 박스에 저장되어 있는 각 Script를 실행하면, 연구자가 작업한 그대로의 output을 분석과정별로(순서대로) 저장하여 공유할 수 있습니다. 이 모든 것을 Data Table 하나에 저장하여, 그 Data Table 하나만 공유하면 됩니다.
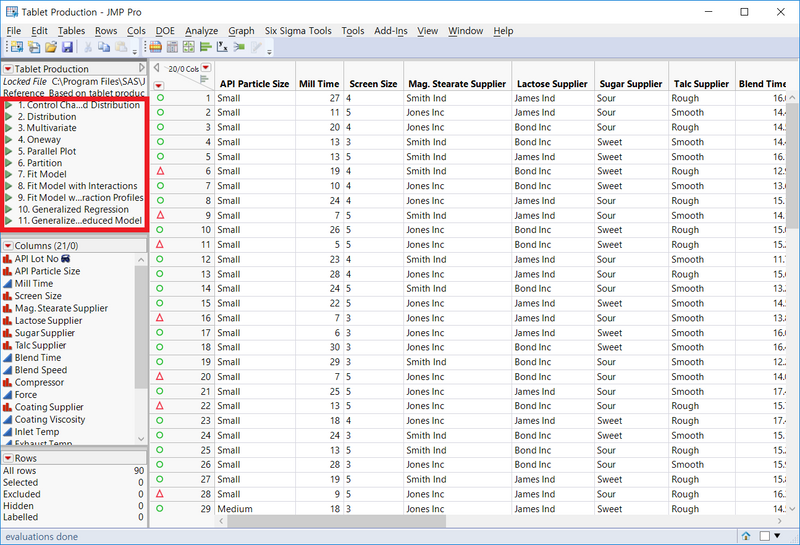
제약/바이오 업계 종사자라면, 2020년 6월부터 적용되는 QbD에 대해 규제기관(MFDS)에 위와 같은 Data Table 하나만 제출하면 되는 것이지요. 또한 규제기관에서도 저장되어 있는 스크립트를 실행함으로써 연구자가 어떤 과정을 거쳐서 어떤 결론에 이르렀는지를 손쉽게 확인하실 수 있습니다.
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.