- JMP User Community
- :
- Blogs
- :
- JMPer Cable
- :
- New Medical Queries: FMQ and AFMQ Implemented in JMP® Clinical
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
The FDA announced the release of the FDA Medical Query (FMQ) and Algorithmic FDA Medical Queries (AFMQ) at the Duke Margolis-FDA Workshop: Advancing Premarket Safety Analytics1 on Sept 14, 2022. The description of FMQ and AFMQ can be found in this FDA presentation: Advancing Premarket Safety Analytics2.
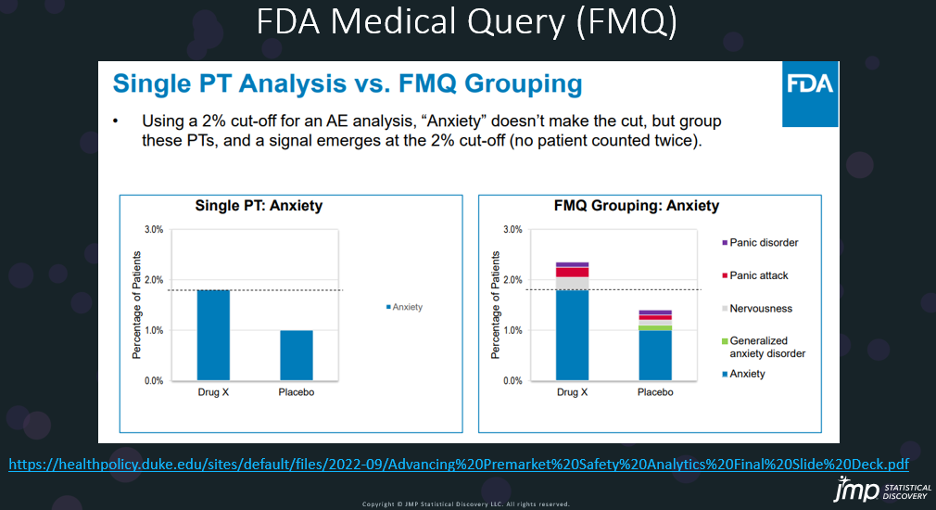
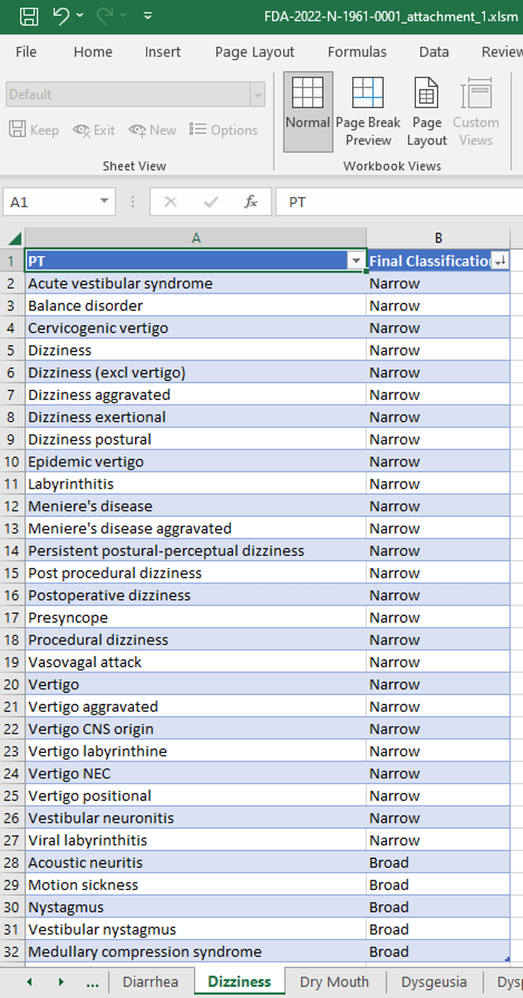
Traditional medical queries (Standard Medical Queries (SMQ))3 focused primarily on MedDRA preferred terms, which clinical trial sponsors used to classify adverse events that occur during the clinical trial. The FMQ consists of groupings of these terms that correlate to specific broader medical conditions. For example, the FMQ for “Dizziness” consists of 31 different MedDRA preferred terms in below Dizziness sheet in the FMQ 2.1 criteria can be downloaded here4. Any trial subject that experiences one or more of these adverse events would be captured as experiencing “Dizziness”. The FMQ version 2.1 has over 100 of these groupings.
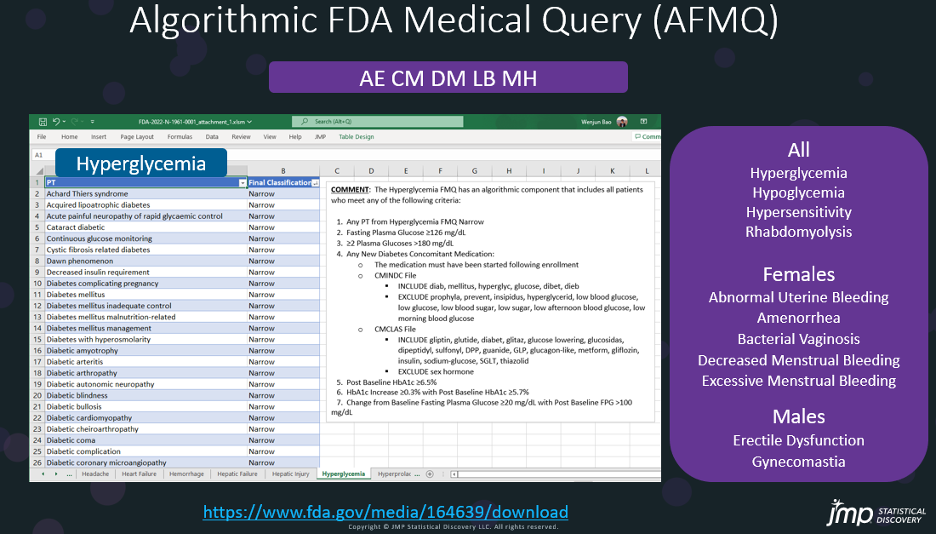
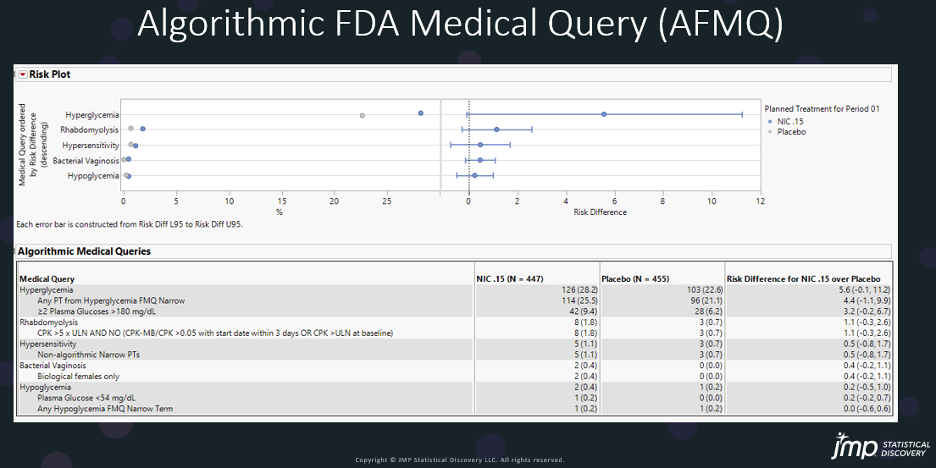
Algorithm FMQs include using additional data from patient demographics, concomitant medications, laboratory findings, and correspondent timing of events with lab results and medications. For example, the AFMQ for Hyperglycemia has multiple criteria as showed below:
|
Hyperglycemia AFMQ Criteria |
|
Any Preferred Term from the Hyperglycemia FMQ (Narrow Terms only) |
|
A Fasting Plasma Glucose ≥126 mg/dL |
|
Two or more Plasma Glucoses >180 mg/dL |
|
Any New Diabetes Concomitant Medication |
|
A Post Baseline HbA1c ≥6.5% |
|
A HbA1c Increase ≥0.3% with Post Baseline HbA1c ≥5.7% |
|
A Change from Baseline Fasting Plasma Glucose ≥20 mg/dL with Post Baseline FPG >100 mg/dL |
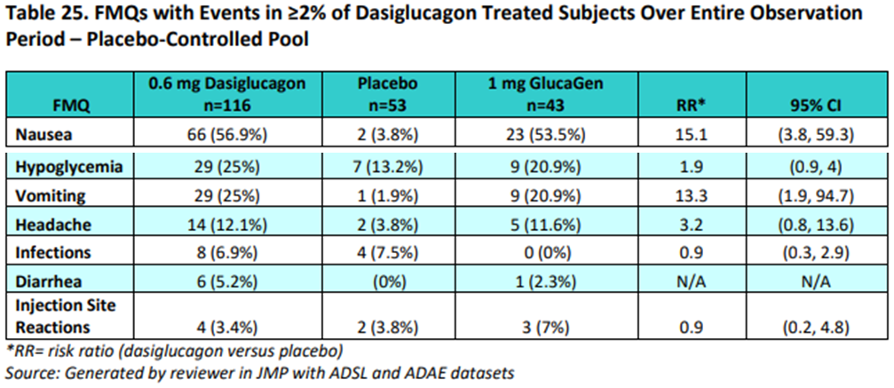
Prior to the release of the FMQ and AFMQ criteria, an FDA reviewer used similar criteria to create a Zegalogue’s Clinical Review (CR) in 20205 to create queries for a FMQ was used by an FDA reviewer to evaluate the common Treatment Emergent Adverse Events (>2%) (shown below). This FMQ was created by JMP, as indicated in the table legend. We mentioned this table but did not show in the Significant AE section in our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation6.
Dr. Veronica Pei, FDA-CDER, presented “Advancing Pre-Market Safety Analytics” at the CDISC Japan Interchange on July 10, 2023, in conjunction with my talk. Her excellent presentation gave a complete overview about the newly released FMQ and AFMQ criteria that made my presentation much easier to understand. In my keynote, “Efficient Evaluation of Clinical Trial Data Following FDA Guides Using FDA NDAs and CRs as Examples,” I included a few slides to talk about how JMP Clinical takes advantage of FMQ, AFMQ, and SMQ to understand drug safety. (In six months, all the CDISC Japan Interchange presentations7 will be available to CDISC members on the CDISC website.)
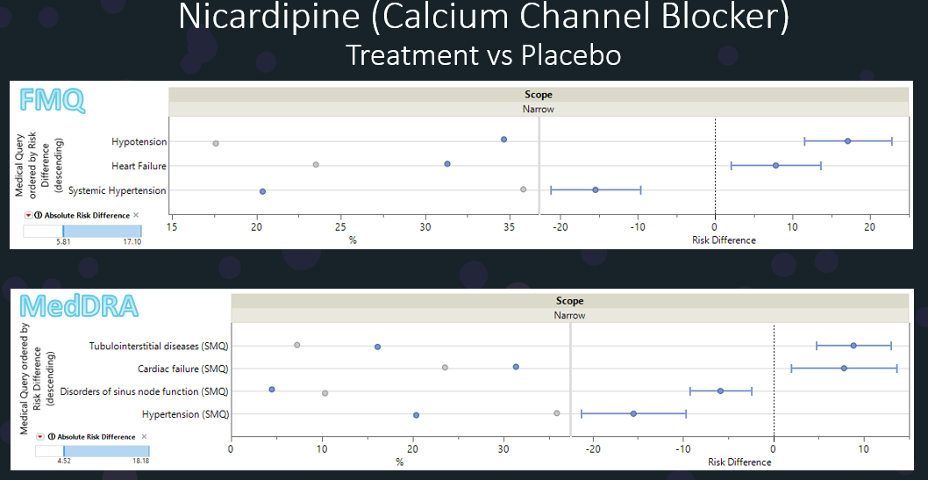
I am sharing a few of my FMQ and AFMQ slides here to show how JMP Clinical delivers the risk report with FMQ and AFMQ.
The FMQ and SMQ (standard medical query) from MedDRA have different formats, terminology, and grouping. FMQ is in Excel with multiple sheets, while SMQ has multiple files in .asc and .seq formats. Currently, the FMQ only supports English, but SMQ supports multiple languages.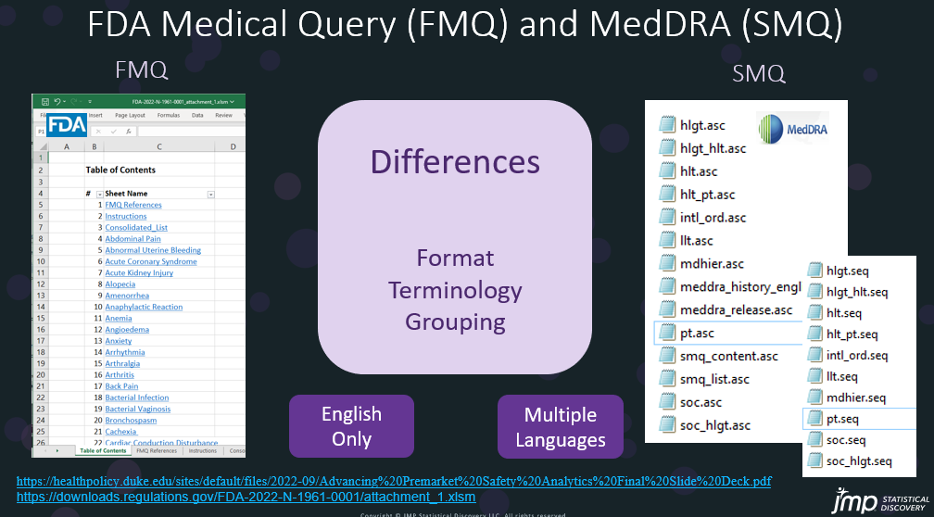
Blog posts in this series:
- New Medical Queries: FMQ and AFMQ Implemented in JMP® Clinical
- Summary of clinical trials using JMP® Clinical
- Safety Results I: Using the Adverse Events Narrative in JMP® Clinical to describe deaths and SAEs
References
- Duke Margolis-FDA Workshop: Advancing Premarket Safety Analytics 2022: https://healthpolicy.duke.edu/sites/default/files/2022-09/Advancing%20Premarket%20Safety%20Analytics...
- FDA Advancing Premarket Safety Analytics 2022: https://healthpolicy.duke.edu/sites/default/files/2022-09/Advancing%20Premarket%20Safety%20Analytics...
- MedDRA https://www.meddra.org/
- FMQ 2.1 download 2022: https://downloads.regulations.gov/FDA-2022-N-1961-0001/attachment_1.xlsm.
- FDA Zegalogue’s Clinical Review 2020 https://www.fda.gov/media/147791/download
- Mann, G. & Pedersen, T. J. & Lyzinski, R. & Scott, A. & Foglia, A. J. & Cromer, J. & Dong, M. & Varga, N. & Gardner, S. & Kirchberg, C. J. & Wingerd, B. A. & Wolfinger, R. D. & Bao, W., (2023) “CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation”, Journal of the Society for Clinical Data Management 3(1). https://doi.org/10.47912/jscdm.169
- CDISC Japan Interchange 2023 https://www.cdisc.org/members-only/interchange-presentations
- FDA Standard Safety Tables and Figures: Integrated Guide 2022 https://www.regulations.gov/document/FDA-2022-N-1961-0046
- © 2024 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- About JMP
- JMP Software
- JMP User Community
- Contact

You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.