- JMP User Community
- :
- Blogs
- :
- JMP Blog
- :
- Generating semi-automated adverse event narratives with the click of a button
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
Adverse event narratives, sometimes called patient narratives or safety narratives, are a summary of each adverse event of every clinical trial participant in a study on an investigational product. They become a critical component of a clinical study report, submitted to regulatory agencies, during the conduct of a clinical trial. Typically, medical writers are responsible for writing them from a collection of tables, listings and figures provided to them from different teams managing the clinical database or reporting on the clinical database.
Why are adverse event narratives useful?
For many years, medical doctors from pharmaceutical sponsors, academics and regulatory authorities have provided guidance describing the contents comprising adverse event narratives to the clinical study report for review by regulatory authorities around the world. The document created from this collection of medical doctors has been available since 1995 provided by the ICHE3 working group document titled “Structure and Content of the Clinical Study Report.” Section 12.3.2 specifically states generating thm for “Narratives of Deaths, Other Serious Adverse Events, and Certain Other Significant Adverse Events.” Serious adverse events are defined in section 12 of the ICHE3 document. This document was adopted by the FDA in July of 1996. For a more comprehensive discussion of adverse event narratives, I recommend a journal article, "Narrative writing: Effective ways and best practices," by Gupta et al. Primarily the focus is on four major categories including: fatalities, non-fatal serious adverse events, non-serious adverse events leading to discontinuation as well as adverse events of special interest. While the first three categories are self explanatory, the fourth category can be anything from adverse events associated with Hy’s Law, adverse events contributing to a Standardized MedDRA Query or about any adverse event deemed a safety issue in a particular class of drugs, etc.
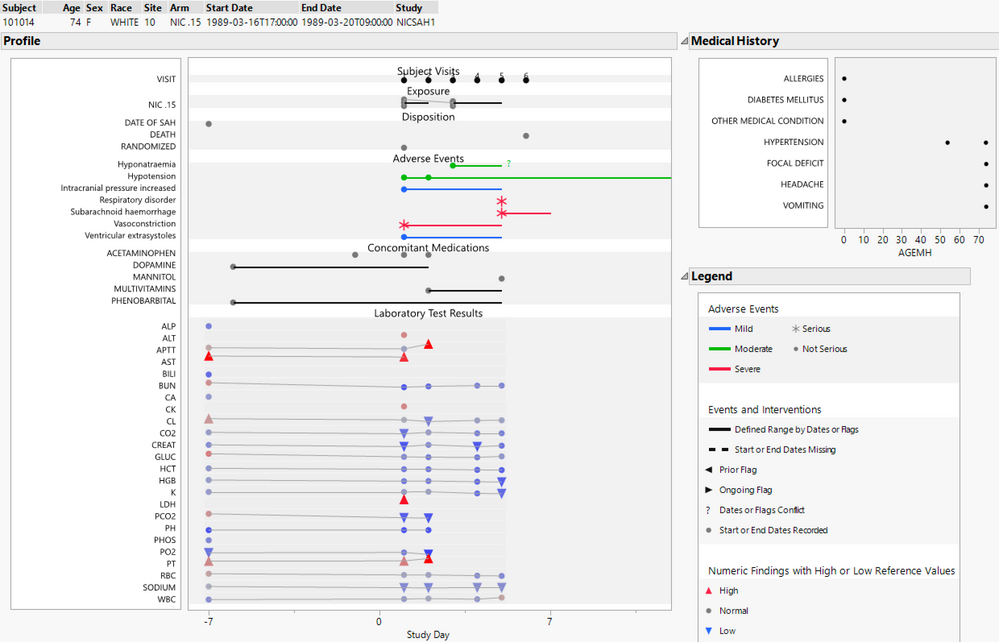
To better understand the content in a narrative, it may help to visualize some of the clinical trial data associated with a subject. The patient profile (shown below) may be used to show adverse events along with other relevant clinical data. It contains much of the same information as in a narrative but in graphical form. I will use subject 101014 as an example throughout this blog post for consistency so that you may recognize the differences in presentation of our semi-automated adverse event narratives. There are quite a few adverse events experienced by the subject, three of which are serious denoted with an asterisk, and two of these are concurrent and classified as fatal.
How are semi-automated adverse event narratives generated?
After gathering the requirements from the guidance documents and input from medical writers on the content of paragraph text, we are ready to start the process of generating the narratives. In order to automate the generation of adverse event narratives, we need access to the clinical database, which is typically the primary data source.
Supplemental sources may include the Council for International Organizations of Medical Sciences forms (CIOMS), Case Report Forms (CRF), MedWatch forms, as well as information from other external systems for tracking adverse event data, concomitant medications as well as exposure data. Many organizations are implementing their clinical database using the Clinical Data Interchange Standards Consortium (CDISC) models such as the Submission Data Tabulation Model (SDTM) https://www.cdisc.org/standards/foundational/sdtmig. JMP Clinical uses most of the domains shown from section 2.5 of the SDTM Implementation Guide below:

CDISC usage at pharmaceutical sponsors has come a long way in the last decade, and many I have met with have a “Data Standards Lead” who helps their organizations utilize data standards for different purposes. Data management teams typically own the clinical database (SDTM data), and biostatistics programming teams own the Analysis Data Model (ADaM) data sets for reporting and analysis purposes.
One very important usage of these CDISC data standards, primarily SDTM and ADaM, can be adverse event narratives, but the requirements for narratives often are not being designed into these models. Therefore, SDTM or ADaM data must be evaluated and potentially augmented to create effective adverse event narratives. If it is not possible to get the teams that manage the SDTM (as well as supplemental qualifers) and/or ADaM data to create the necessary variables, then a third party must take ownership of this process. Since the SDTM and ADaM variability within an organization can still be high, it is necessary to make decisions about how much work should be done to harmonize the requirements of the medical writers versus delivering narrative features immediately for the sake of time. JMP Clinical is a very helpful tool to help you evaluate your data for readiness with semi-automated narrative generation and deliver out-of-the-box features immediately until there is time to modify the data and code to create the perfect adverse event narrative. In the end, it may not be necessary to reach perfection as we are able to merge any supplemental data from any of the databases with use of an optional supplemental data table input field for the adverse event narratives.
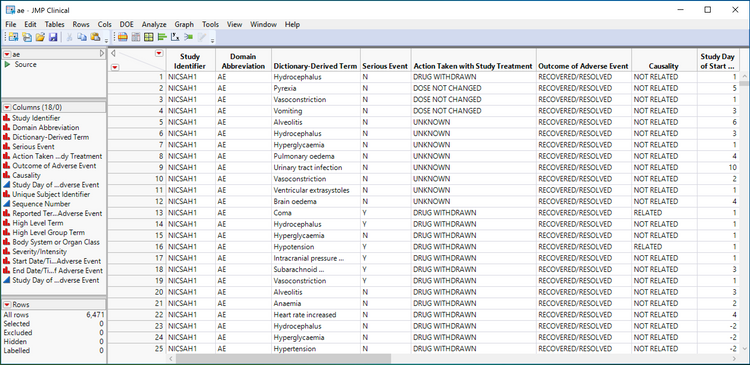
Shown above is a JMP table view of a SAS transport file for the CDISC SDTM AE domain. Notice the column names (technically, they are labels) in the above adverse event table. Serious Event, Action Taken with Study Treatment, Causality and Outcome of Adverse Event are all visible and indicated as required information in the guidance documents described above for tracking adverse events that occur during Phase I, II, III and IV of clinical trials.
How many types of adverse event narratives can we provide?
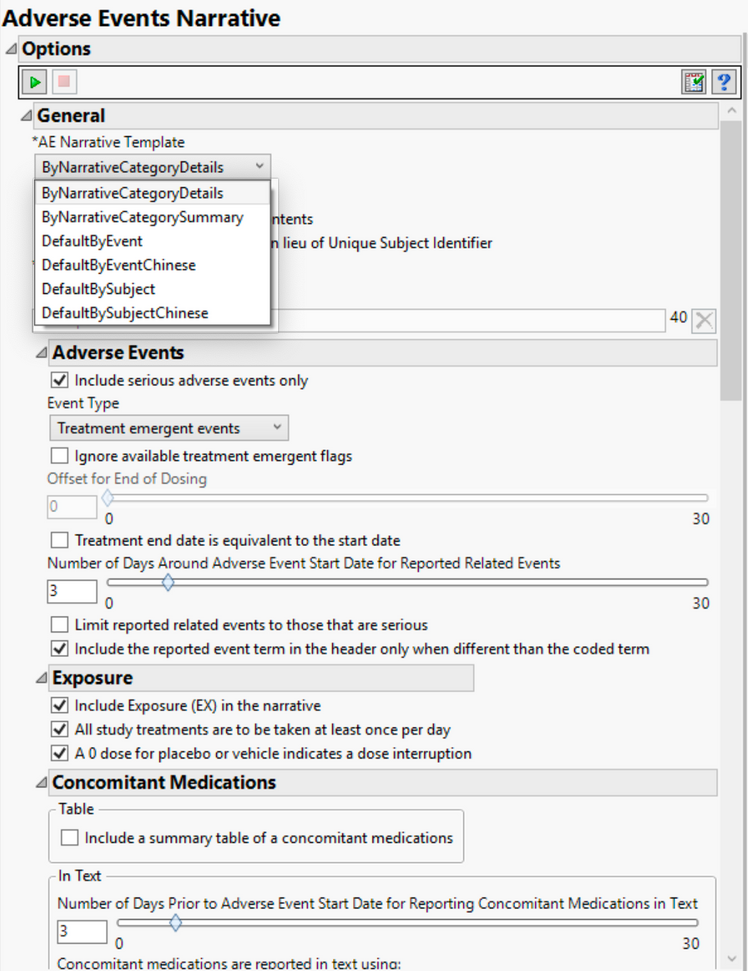
In a much earlier version of JMP Clinical, version 4, we introduced the ability to create adverse event narratives with options to select whether the user just wanted to print serious adverse events or all all adverse events as well as deciding whether to just print treatment emergent events or pre-treatment, post-treatment, etc. These options, available from dialogs (as shown below) in the software, provided flexibility for the end user to decide which content they preferred in their safety narratives. Now with JMP Clinical 7.1, these options have increased due to customer requests.
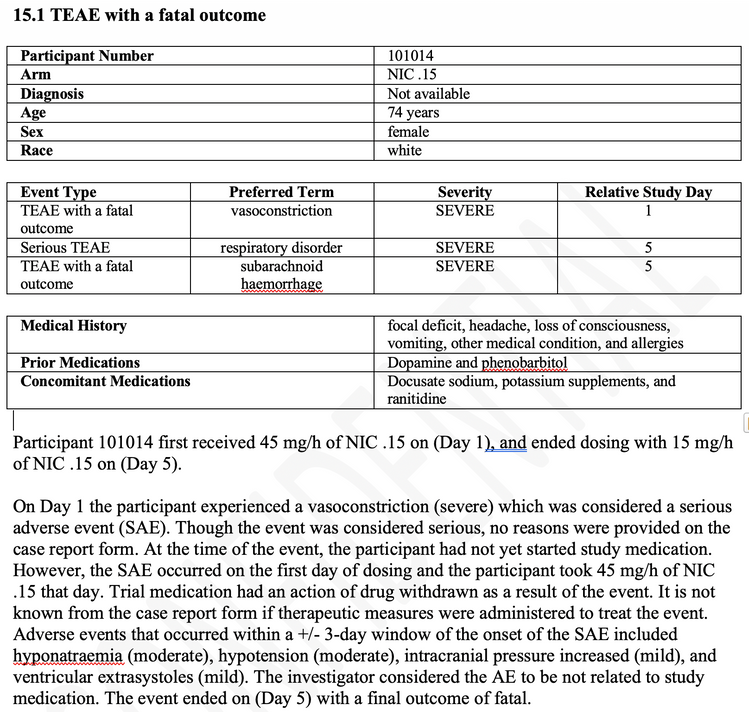
When selecting the “DefaultByEvent” template with one of the selected studies in JMP Clinical, the following example is generated. Each event, in this case a fatal subarachnoid haemorrhage, is printed in the following manner. A header displays demographic and exposure details, followed by a heading in bold of the adverse event. The first paragraph provides more detail of demographics, medical history, exposure and outcome. The second paragraph describes the adverse event in detail, with the exposure at the time of the event, followed by the action taken with the drug. The third paragraph describes the other adverse events that occurred within a specified number of days (window around the current event) as well as the medications taken within a similar or different time window. The last paragraph details the causality and outcome of the adverse event. Not shown are optional paragraphs to detail laboratory or other findings data details relevant to the adverse event.

This example, while a little updated with newer features, was the first type of narrative requested by customers. Over the following years, it became clear that many of our customers preferred to group all of the events that a participant exhibited under one header in the order that they occurred relative to study day 1. The next example below shows this approach with our “DefaultBySubject” template. For comparison, I use the same patient as in the above example, so that you can see the relative differences in organization under a single patient header in grey.

Upon seeing these more organized "by subject" narratives, customers asked for a clear and concise summary of each participant using tables formatted to indicate 1) demographic information, 2) adverse events sorted by severity of event type and then study day, and finally another table for medical history with concomittant medications and additional information for oncology therapies.
Notice that participant number 101014, shown in the examples above, falls into multiple adverse event categories and will appear in the fatality section of the document since they had at least one fatal adverse event. Customers have requested that we start organizing the adverse event narrative document by the categories of fatal, serious but not fatal, non-serious leading to discontinuation and special interest. These features get us one step closer towards semi-automating the entire adverse event narrative section of the clinical study report. In order to accomplish this, we need to summarize all participants with an event in a way that the reader can quickly assess them and the categories into which they fall. See the example below:
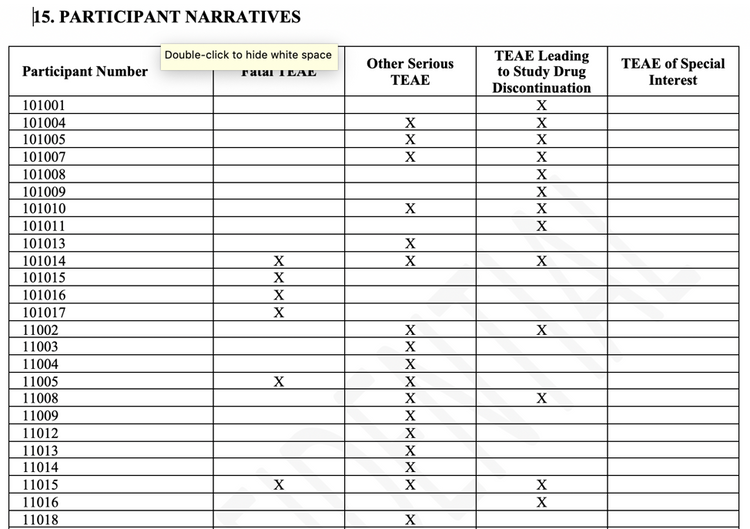
The latest version, JMP Clinical 7.1, which was released in August 2019, contains even more new features of adverse event narratives with built-in table views of data than shown above. Customized tables of any laboratory data or other data is now possible with a single line of code. Using our new automation API to load studies into the system and run reports, you may not have to click a single button to generate adverse event narratives again. However if you are having to triage questions from regulatory authorities and generate adverse event narratives for subjects that had not been included in the clinical study report, it is possible to use any visualization in our system to quickly generate these with the click of a button.
To learn more about automating adverse event narratives, sign up to watch the upcoming webinar hosted DIA.
- © 2024 JMP Statistical Discovery LLC. All Rights Reserved.
- Terms of Use
- Privacy Statement
- About JMP
- JMP Software
- JMP User Community
- Contact

You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.