- User Community
- :
- JMP Discovery Summit Series
- :
- Past Discovery Summits
- :
- Discovery Summit Europe 2019 Presentations
- :
- Using JMP(R) for Statistical Analysis of Biological Assays and Test ( 2019-EU-45...
Level: Intermediate
Job Function: Analyst / Scientist / Engineer
Donald McCormack, JMP Technical Enablement Engineer, SAS
Statistical analysis of biological assays and tests is commonly carried out in the pharmaceutical and biotechnology industries. It is an important component to regulatory submissions, development of standard operating procedures and maintenance of good manufacturing practices. Standards are frequently employed for carrying out these procedures and in Europe, Section 5.3 of the European Pharmacopoeia (Statistical Analysis of Results of Biological Assays and Tests) – EP 5.3 – is possibly the most often used standard. While EP 5.3 and similar documents are invaluable in providing guidance for performing complex and detailed procedures, there are often drawbacks. EP 5.3 relies on hand calculated values for all statistical estimates which are, unless operationalized via computer algorithm, prone to error. In addition, the formulas provided are not necessarily generalizable to other situations, such as when the design for data collection is unbalanced. The simplifications used in formula development may also lead to inconsistencies in the statistical estimates.
In this presentation JMP will be used to demonstrate how the analyses in EP 5.3 can be carried out quickly, consistently, and accurately. Examples from the document will be used to illustrate this process. Inconsistencies in EP 5.3 will be discussed and alternative methods for analysis will be presented. A JSL script for the calculating of relative potency and its confidence interval will also be demonstrated.
Hello Don,
Good work.
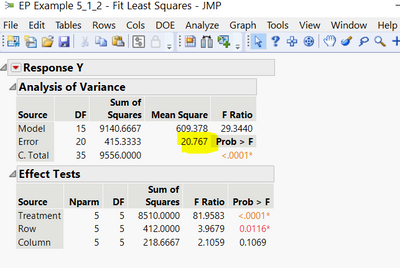
However for the EP user, these examples are very difficult to follow for actual average users in the quality control. If the example 5.1.2 could explain how the various parameters such as Ps, PT, HL and the final estimation of potency was calculated using the JMP, then that would be great.
For a QC lab to buy JMP to do these calculations, the use should have been explained in greater detail.
I will be obliged if some one could explain the example 5.1.2 in greater detail step by step.
Regards
Hello,
Its not clear how this was calculated:
@peerr12, thanks for your question. JMP is using the standard approach to calculating sums of squares and mean squares as suggested by Example 5.1.2 of the European Pharmacopoeia 6.0 (suggested, as the example uses hand calculations which are equivalent to the computational results when the data is balanced, i.e., the same number of observations for each cell in the Latin Square). In general, we're calculating the variability attributed to the model and comparing it to the error (unexplained) variability. If this ratio is large, there's reason to believe the model is more than just noise. We're working with mean squares so everything is scaled by the amount of information that goes into creating each statistic and things are comparable. A more detailed version of what goes on and the calculations involved with analysis of variance (ANOVA), can be found here. The explanation spans a few pages. You can also find similar material in most introductory statistics books, but the NIST/Sematech Handbook tries to minimize the statistical jargon and explain things from the viewpoint of a non-statistician.
- Subscribe to RSS Feed
- Mark as New
- Mark as Read
- Bookmark
- Subscribe
- Printer Friendly Page
- Report Inappropriate Content
- © 2025 JMP Statistical Discovery LLC. All Rights Reserved.
- Cookie Preferences
- Terms of Use
- Privacy Statement
- Contact Us