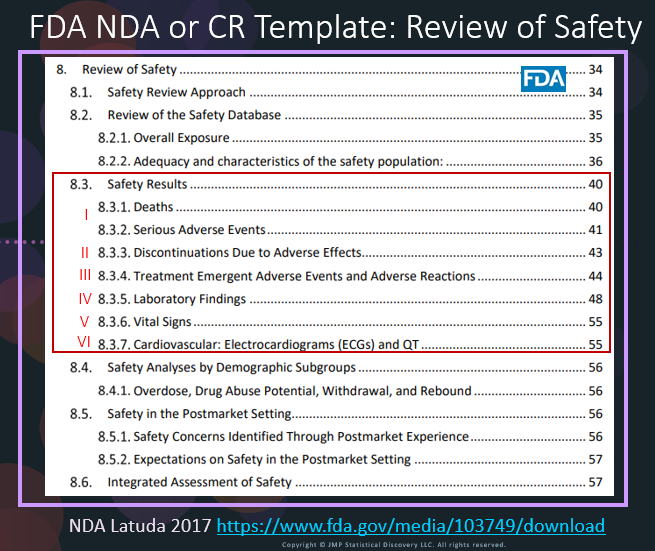
In our recent paper, CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation1,2, we followed FDA NDA (New Drug Application) or CR (Clinical Reviews) templates to show how drug safety can be evaluated. My blog, Safety Result I, explained how to use the Adverse Events Narrative to summarize the individual information3 for those subjects that had a SAE in CR for Latuda4. The below figure is from Latuda’s4 CR content, which lists the detailed aspects in the Safety Results section. Our paper1 and my blogs follow these steps to evaluate the drug safety.
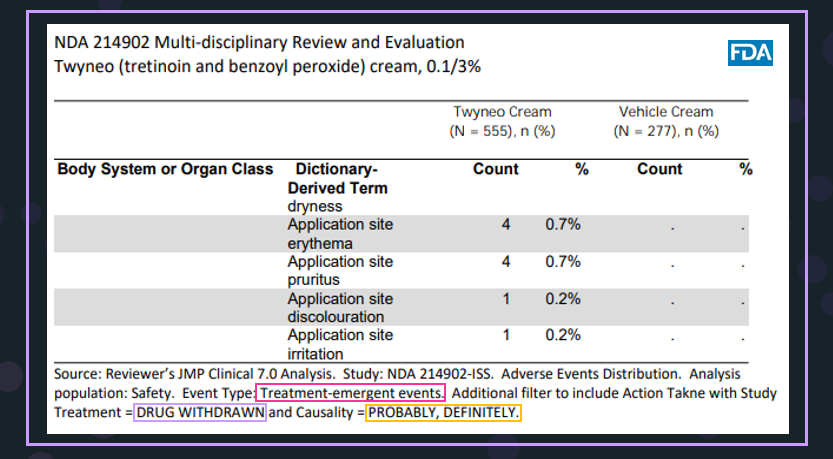
The second step in Safety Results is to understand the discontinuations due to adverse effects as shown in the table from the NDA for Twyneo5. In this table's legend, the reviewer clearly stated that the options in Adverse Events Distribution in JMP Clinical were used to obtain the results shown in the table below. The specific options are framed in different colors.
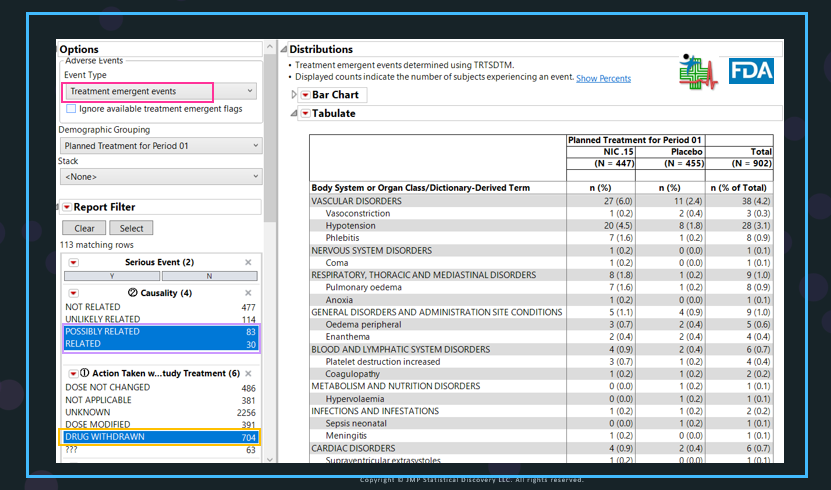
The options in the Adverse Events Distribution report in JMP Clinical for assessing the discontinuations or drug withdrawn due to AEs using the Nicardipine sample data were set up as follows:
- In the Adverse Events Distribution, the default Analysis Population setting is Safety, which can be changed with the options listed in Set Study Preference.
- For Event Type, Treatment-emergent events (framed in red) was selected. For the Causality, Possibly Related and Related for Nicardipine were chosen, which are similar to Probably and Definitely for Twyneo5, framed in purple.
- Action Taken with Study Treatment, framed in yellow in the left panel of the AE Distribution report, was selected for Drug Withdrawn.
The content lists in Causality and Action Taken with Study Treatment are based on the data. The table was generated following FDA Standard Safety Tables and Figures: Integrated Guide6 2022.
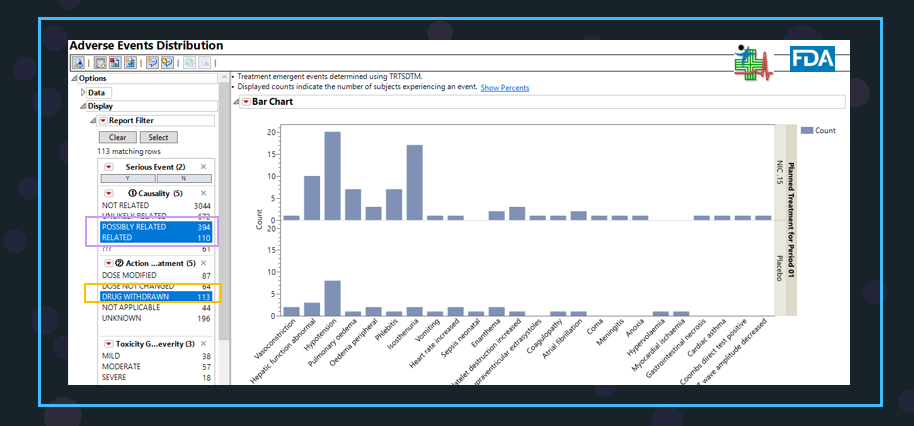
The same results can also be displayed in the distribution plot.
References:
- Mann, G. & Pedersen, T. J. & Lyzinski, R. & Scott, A. & Foglia, A. J. & Cromer, J. & Dong, M. & Varga, N. & Gardner, S. & Kirchberg, C. J. & Wingerd, B. A. & Wolfinger, R. D. & Bao, W., (2023) “CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation”, Journal of the Society for Clinical Data Management 3(1). https://doi.org/10.47912/jscdm.169
- CDISC Enables Efficient Streamlining of Clinical Trial Safety Evaluation published in JSCDM
- Safety Results I: Death and SAE described by AE Narrative in JMP Clinical
- CR Latada 2018 https://www.fda.gov/media/103749/download
- NDA Twyneo 2020 https://www.fda.gov/media/151645/download
- FDA Standard Safety Tables and Figures: Integrated Guide 2022 https://www.regulations.gov/document/FDA-2022-N-1961-0046
You must be a registered user to add a comment. If you've already registered, sign in. Otherwise, register and sign in.